Continuous in situ electrogenaration of a 2-pyrrolidone anion in a microreactor: application to highly efficient monoalkylation of methyl phenylacetate†
Abstract
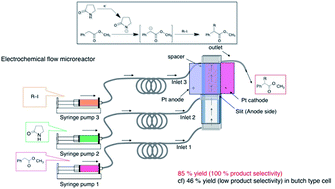
We have successfully demonstrated effective generation of an electrogenerated base (EGB) such as the 2-pyrrolidone anion and its rapid use for the following alkylation reaction in a flow microreactor system without the need for severe reaction conditions. The key feature of the method is effective and selective preparation of monoalkylated products.

 Please wait while we load your content...
Please wait while we load your content...