Simultaneous fluoroimmunoassay of two tumor markers based on CdTe quantum dots and gold nanocluster coated-silica nanospheres as labels†
Abstract
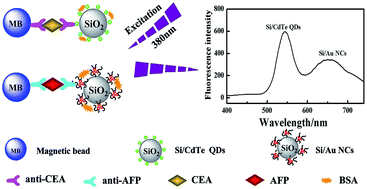
In this study, a novel fluoroimmunoassay protocol for simultaneous detection of two tumor markers is described. The new approach employed magnetic beads as a carrier for the antibody immobilization, while CdTe quantum dots (CdTe QDs) and gold nanoclusters (Au NCs) coated-silica nanospheres were used as labels. Carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) were adopted as model proteins. After a typical sandwich-type immunoreaction, the immunocomplex exhibited two distinguishable fluorescence peaks at 550 nm and 655 nm corresponding to CdTe QDs and Au NCs, respectively. Under optimal conditions, fluorescence intensities were linearly increased to the concentration of CEA and AFP in the range of 0.1–400 ng mL−1, the detection limit of fluoroimmunoassay is 0.04 ng mL−1 for CEA and 0.08 ng mL−1 for AFP, respectively. The proposed method was evaluated with human serum, and the determination values obtained were in accordance with reference methods reported. These results demonstrated that the new method can be applied to the determination of two tumor markers in clinical samples.

 Please wait while we load your content...
Please wait while we load your content...