Fabrication and biocompatibility of reduced graphene oxide/poly(vinylidene fluoride) composite membranes
Abstract
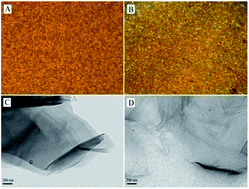
The graphene/polymer composite membranes were prepared by an in situ thermal reduction method and their biocompatibility was evaluated. Brown graphite oxide (GO)/poly(vinylidene fluoride) (PVDF) composites were initially prepared by solution processing, then converted to black membranes through compression molding at 200 °C. Structural analyses indicated that GO was reduced and well dispersed in PVDF matrix during the processing course, and revealed a wrinkled topography of the reduced graphene oxide (RGO). Incorporation of RGO facilitated the formation of β phase PVDF and increased its wettability. Cell culture results showed that human umbilical vein endothelial cells (HUVECs) spread and proliferated much better on the composite membranes, indicative of promoted cell adhesion and proliferation and enhanced biocompatibility of the membranes. Such excellent performance of the RGO/PVDF composite membranes makes them promising candidates as biomaterials.

 Please wait while we load your content...
Please wait while we load your content...