Isoindigo-based microporous organic polymers for carbon dioxide capture†
Yang Zhao,
Xiaoyan Wang,
Chong Zhang,
Fangyuan Xie,
Rui Kong and
Jia-Xing Jiang*
Key Laboratory for Macromolecular Science of Shaanxi Province, School of Materials Science and Engineering, Shaanxi Normal University, Xi’an, Shaanxi 710062, P. R. China. E-mail: jiaxing@snnu.edu.cn
First published on 10th November 2015
Abstract
Isoindigo-based microporous organic polymers from nitrogen- and oxygen-rich 6,6′-dibromoisoindigo and its alkylated derivatives have been synthesized via a palladium-catalyzed Sonogashira–Hagihara cross-coupling reaction. The pore properties (pore size & surface area) of this kind of microporous organic polymers could be tuned by the alkyl groups connected to the 6,6′-dibromoisoindigo unit. Owing to the incorporation of nitrogen atoms and ketonic groups from the isoindigo unit into the skeleton of the microporous polymers, enhancing the binding affinity between the pore wall and CO2 molecules, the polymers show high isosteric heats of CO2 adsorption of 27.4–33.5 kJ mol−1, which are higher than those of many reported porous organic polymers. Compared with the alkylated polymers of TBMIDM and TBMIDE, TBMID without an alkyl group exhibits a high CO2 uptake ability of 3.30 mmol g−1 (1.13 bar/273 K) with a CO2/N2 sorption selectivity of 58.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, because of the strong interactions between the polymer network and the polarizable CO2 molecules through dipole–quadrupole interactions and/or hydrogen bonding. These data demonstrate that these isoindigo-based microporous organic polymers could be potential candidates for applications in post-combustion CO2 capture and sequestration.
1, because of the strong interactions between the polymer network and the polarizable CO2 molecules through dipole–quadrupole interactions and/or hydrogen bonding. These data demonstrate that these isoindigo-based microporous organic polymers could be potential candidates for applications in post-combustion CO2 capture and sequestration.
1. Introduction
Carbon dioxide is deemed to be a significant contribution to global warming and some environmental issues. Developing highly efficient carbon capture and separation technologies or CO2 capture materials are potential strategies to address these issues. Amine-based wet scrubbing technology for CO2 capture suffers from the considerable energy penalty for CO2 release and the regeneration of amine solution.1 Porous solids, which adsorb CO2 molecules through relatively weak van der Waals forces, are emerging as a new class of CO2 capture materials. A range of porous solids have been proposed for the capture of carbon dioxide, which mainly include microporous zeolites,2 activated carbons,3 metal organic frameworks (MOFs),4 porous organic molecules,5,6 and microporous organic polymers (MOPs).7,8 Among of them, MOPs have attracted an increasing amount of recent attention because they show great potential in a variety of applications such as gas storage and separation,9,10 heterogeneous catalysis11,12 and chemical sensors.13,14 To date, a wide range of MOPs have been developed by using various building blocks and synthetic strategies. These mainly include polymers of intrinsic microporosity (PIMs),15 covalent organic frameworks (COFs),16,17 conjugated microporous polymers (CMPs),18–20 porous polymer networks (PPNs),21 porous aromatic frameworks (PAFs),22 covalent triazine-based frameworks (CTFs),23 and hypercrosslinked porous polymers (HCPs).24–26 However, most reported MOPs without polar groups or heteroatoms usually showed a low CO2 adsorption capacity at ambient temperatures and pressures because of the lack of strong CO2 binding sites. Recent studies have revealed that electron-rich porous polymer networks can yield strong dipole–quadrupole interactions with CO2 molecules, leading to a significant increase in the CO2 capture capacity, and thus a range of nitrogen-rich and/or oxygen-rich microporous organic polymers have been synthesized for the capture of CO2. For example, the microporous polycarbazole of CPOP-1 with a high nitrogen content showed a CO2 capture capacity of 4.82 mmol g−1 and a CO2/N2 adsorption selectivity of 25 at 273 K/1.0 bar.27 The imine-linked porous polymer framework of PPF-1 exhibited a CO2 uptake of 6.1 mmol g−1 with a CO2/N2 adsorption selectivity of 14.5 at 273 K/1.0 bar.28 The benzimidazole-linked polymer of BILP-4 could adsorb CO2 up to 5.3 mmol g−1 with a CO2/N2 adsorption selectivity of 79 at 273 K/1.0 bar.29 The nanoporous azo-linked polymer of ALP-1 showed a CO2 uptake of 5.36 mmol g−1 with a CO2/N2 adsorption selectivity of 35 at 273 K/1.0 bar.30 The porous triazine bifunctionalized task-specific polymer of TSP-2 could adsorb CO2 up to 4.1 mmol g−1 with a CO2/N2 adsorption selectivity of 38 at 273 K/1.0 bar.31 The nanoporous covalent organic polymer with Tröger’s base functionality of TB-COP-1 exhibited an enhanced CO2 capture capacity of 5.19 mmol g−1 with a CO2/N2 adsorption selectivity of 79.2 at 273 K.32 Iron-containing porphyrin porous organic polymers showed a high CO2 uptake ability of 4.30 mmol g−1 at 273 K/1.0 bar,33 and the triazine-functionalized porphyrin-based porous organic polymer of TPOP-1 exhibited a CO2 uptake as high as 6.2 mmol g−1 at 273 K/3.0 bar.34 These results demonstrated that such electron-rich MOPs have great potential to increase the CO2 uptake ability or CO2/N2 adsorption selectivity. Palladium-catalyzed Sonogashira–Hagihara cross-coupling polymerization has been widely used to produce a range of highly porous organic polymers. For example, the conjugated microporous poly(aryleneethynylene) networks synthesized by Sonogashira–Hagihara cross-coupling polymerization showed a high surface area up to 834 m2 g−1.18 CMPs with a range of chemical functionalities, including carboxylic acids, amines, hydroxyl groups and methyl groups, exhibited high isosteric heats of sorption for CO2.35 The metal–organic conjugated microporous polymers produced by a Sonogashira–Hagihara coupling reaction could be used as a heterogeneous catalyst.36 Conjugated nanoporous polymer colloids were synthesized by using a Sonogashira–Hagihara coupling reaction.37 Cobalt/aluminium-coordinated CMPs exhibited outstanding CO2 capture capacity and conversion performance at atmospheric pressure and room temperature.38Isoindigo is an efficient acceptor species that has been studied widely in the areas of organic photovoltaic cells39 and dye-sensitized solar cells.40 However, to the best of our knowledge, there are no reports on the synthesis of porous polymers based on isoindigo or its derivatives. We present here the synthesis of a novel class of isoindigo-based MOPs via a one-pot palladium-catalyzed Sonogashira–Hagihara cross-coupling reaction. Isoindigo contains both nitrogen and oxygen atoms, making the resulting porous polymer highly electron-rich, which could enhance the binding affinity between the adsorbent and CO2 molecules, and thus we hypothesized that the introduction of isoindigo segments into the porous polymer skeleton could lead to an increase in the CO2 capture capacity. In addition, we also synthesized isoindigo-based MOPs with different alkyl substituents, and investigated the influence of the alkyl substituent on the porosity and CO2 capture capacity of the resulting porous polymers.
2. Experimental section
2.1 Chemicals
6-Bromooxindole and 6-bromoisatin were purchased from TCI. Triphenylphosphine, tetraphenylmethane, trimethylsilylacetylene and copper(I) iodide were purchased from Alfa. K2CO3 and bromoethane were purchased from Acros. Iodomethane, tetrakis(triphenylphosphine)palladium(0), bis(triphenylphosphine)palladium(II) chloride, N,N-dimethylformamide, triethylamine and other chemicals were purchased from J&K Scientific Ltd. All chemicals were used as received. 6,6′-Dibromoisoindigo, 6,6′-dibromo-N,N′-(2-methyl)-isoindigo, 6,6′-dibromo-N,N′-(2-ethyl)-isoindigo39 and tetra(4-ethynylphenyl)methane41 were prepared according to previously reported methods.2.2 Synthesis of isoindigo-based microporous organic polymers
All of the polymer networks were synthesized by a palladium(0)-catalyzed Sonogashira–Hagihara cross-coupling reaction of 6,6′-dibromoisoindigo, 6,6′-dibromo-N,N′-(2-methyl)-isoindigo or 6,6′-dibromo-N,N′-(2-ethyl)-isoindigo with tetra(4-ethynylphenyl)methane. All reactions were carried out at a fixed total molar monomer concentration of 75 mmol L−1 and a fixed reaction temperature and reaction time (100 °C/48 h). A representative experimental procedure for TBMID is given as an example.2.3 Characterization
The FT-IR spectra were measured in transmission mode on a Tensor 27 FT-IR spectrometer (Bruker) using KBr disks. The thermal properties of the polymer networks were evaluated using thermogravimetric analysis (TGA) with a differential thermal analysis instrument (Q1000DSC + LNCS + FACS Q600SDT) over the temperature range from 30 to 800 °C under a nitrogen atmosphere with a heating rate of 10 °C min−1. The morphologies of the polymer networks were obtained using a field emission scanning electron microscope (SEM, JSM-6700F, JEOL, Japan) and a high resolution transmission electron microscope (TEM, JEM-2100F, JEOL, Japan). Powder X-ray diffraction measurement was carried out on an X-ray Diffractometer (D/Max-3c). Solid state magic angle spinning 13C CP/MAS NMR measurements were carried out on a Bruker Avance III model 400 MHz NMR spectrometer at a MAS rate of 5 kHz. Elemental analysis was performed on a EURO EA3000 Elemental Analyzer. Surface areas and pore size distributions were measured by nitrogen adsorption and desorption at 77.3 K using an ASAP 2420-4 (Micromeritics) volumetric adsorption analyzer. The surface areas were calculated in the relative pressure (P/Po) range from 0.05 to 0.20. Pore size distributions and pore volumes were derived from the N2 adsorption branch of the isotherms using non-local density functional theory (NL-DFT). The samples were degassed at 120 °C for 15 h under vacuum (10−5 bar) before analysis. The gas sorption isotherms were measured on an ASAP 2420-4 as well.3. Results and discussion
Scheme 1 shows the general synthetic route for the three isoindigo-based microporous organic polymers. 6,6′-Dibromoisoindigo and its alkylated derivatives with methyl or ethyl groups were employed as building blocks in order to study the influence of alkyl groups on the pore properties and gas adsorption performances of the resulting polymers. Tetra(4-ethynylphenyl)methane was selected as the comonomer since it possesses four reactive sites, which makes the polymers highly crosslinked molecular structures. All of the polymer networks were insoluble in common organic solvents such as THF, DMF, CHCl3 and methanol. The resulting polymer networks showed high thermal stability in a nitrogen atmosphere, as revealed by TGA (up to 350 °C, Fig. S1†), and were also chemically stable. The FT-IR spectra of the polymer network (Fig. S2†) structures show the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration bands at 1600 cm−1, the alkyne of –C
C vibration bands at 1600 cm−1, the alkyne of –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– band at 2200 cm−1, the stretching vibration band of C–N–C at 1494 cm−1, and C
C– band at 2200 cm−1, the stretching vibration band of C–N–C at 1494 cm−1, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations at around 1705 cm−1 in all the samples. In addition, TBMID, without any alkyl substituent, showed an N–H stretching vibration at around 3213 cm−1 and a bending vibration at 1312 cm−1, which were not observed for TBMIDM (with methyl group) and TBMIDE (with ethyl group). The –CH3 stretching vibration at around 2925 cm−1 and the bending vibration 1375 cm−1 could be detected for TBMIDM. The –CH2–, –CH3 stretching vibration at around 2930 cm−1, 2973 cm−1 and the bending vibration 1347 cm−1, 1371 cm−1 could be detected respectively for TBMIDE. The polymer networks were also characterized at the molecular level by solid state 1H–13C cross-polarization magic-angle spinning (CP/MAS) NMR spectroscopy to further confirm the structure of the polymer networks. The 1H–13C CP/MAS NMR spectra and the assignment of the resonances are shown in Fig. 1. All of the polymer networks showed characteristic peaks at approximately 169 ppm (–C
O stretching vibrations at around 1705 cm−1 in all the samples. In addition, TBMID, without any alkyl substituent, showed an N–H stretching vibration at around 3213 cm−1 and a bending vibration at 1312 cm−1, which were not observed for TBMIDM (with methyl group) and TBMIDE (with ethyl group). The –CH3 stretching vibration at around 2925 cm−1 and the bending vibration 1375 cm−1 could be detected for TBMIDM. The –CH2–, –CH3 stretching vibration at around 2930 cm−1, 2973 cm−1 and the bending vibration 1347 cm−1, 1371 cm−1 could be detected respectively for TBMIDE. The polymer networks were also characterized at the molecular level by solid state 1H–13C cross-polarization magic-angle spinning (CP/MAS) NMR spectroscopy to further confirm the structure of the polymer networks. The 1H–13C CP/MAS NMR spectra and the assignment of the resonances are shown in Fig. 1. All of the polymer networks showed characteristic peaks at approximately 169 ppm (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 144 ppm (N–Car), 110 ppm (–C
O), 144 ppm (N–Car), 110 ppm (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 92 ppm (–C
C–), 92 ppm (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–) and 65 ppm, corresponding to the quaternary carbon atom that is connected to four phenyl groups. In addition, the signal at approximately 26 ppm from –CH3 was observed for TBMIDM, and the signals at approximately 35 ppm (–CH2–) and 12 ppm (–CH3) were also observed for TBMIDE, while these peaks were not observed in the 1H–13C CP/MAS NMR spectrum of TBMID. These results are consistent with the expected polymer networks. No clear diffraction peaks could be observed in the powder X-ray diffraction profiles (Fig. S3†), indicating that the polymer networks are amorphous in nature. Scanning electron microscopy images revealed that the three polymers possess very similar morphologies with relatively uniform solid submicrometer spheres (Fig. S4†), indicating that the alkyl group has a negligible influence on the morphology of the resulting polymers. High resolution transmission electron microscopy (HR-TEM) images also demonstrated the amorphous structure of the polymer networks and the presence of homogenous pores in the polymers (Fig. S5†).
C–) and 65 ppm, corresponding to the quaternary carbon atom that is connected to four phenyl groups. In addition, the signal at approximately 26 ppm from –CH3 was observed for TBMIDM, and the signals at approximately 35 ppm (–CH2–) and 12 ppm (–CH3) were also observed for TBMIDE, while these peaks were not observed in the 1H–13C CP/MAS NMR spectrum of TBMID. These results are consistent with the expected polymer networks. No clear diffraction peaks could be observed in the powder X-ray diffraction profiles (Fig. S3†), indicating that the polymer networks are amorphous in nature. Scanning electron microscopy images revealed that the three polymers possess very similar morphologies with relatively uniform solid submicrometer spheres (Fig. S4†), indicating that the alkyl group has a negligible influence on the morphology of the resulting polymers. High resolution transmission electron microscopy (HR-TEM) images also demonstrated the amorphous structure of the polymer networks and the presence of homogenous pores in the polymers (Fig. S5†).
 | ||
| Fig. 1 Solid-state 1H–13C CP-MAS NMR spectra of the polymer networks, the asterisks denote spinning side bands. | ||
The porosity of the polymer networks was measured by nitrogen adsorption and desorption analyses at 77.3 K. As shown in Fig. 2a, all of the resulting polymer networks gave rise to typical type-I nitrogen gas sorption isotherms according to IUPAC classification,42 indicating that these polymer networks are microporous in nature. This was also evidenced by the pore size distribution (PSD) curves for the polymer networks, as calculated using non-local density functional theory (NL-DFT). All of the polymer networks exhibited an abundant micropore structure with pore sizes of less than 2.0 nm (Fig. 2b). Compared with TBMIDM and TBMIDE, TBMID without any substitution exhibited a smaller micropore size centered at around 0.9 nm. TBMIDM showed a micropore size centered at around 1.1 nm and TBMIDE exhibited a micropore size centered at 1.0 nm, indicating that the pore size of the polymer networks could be controlled by the substituent group, as observed in other reported CMPs with alkyl substituents.43 Unlike the crystalline MOFs and COFs, where the pore size could be finely narrowed by increasing the size of the substituent group grafted on the ligand or the building block,44–46 these amorphous polymers do not show a simple and rational pore size order upon increasing the size of the substituent group from H to CH3 to CH2CH3. For example, TBMID, without any alkyl group, shows the smallest pore size of 0.9 nm, while TBMIDM, with methyl groups, shows the biggest pore size of 1.1 nm, as shown in Fig. 2b. We do not at present have a full explanation for this result, but it could be partially attributed to the increased distance among the polymer chains because of the increasing steric hindrance going from H to CH3 to CH2CH3, which hinders the polymer’s ability to pack into small pores during polymerization; a similar result was observed for other reported CMPs with different alkyl chains.47 The apparent Brunauer–Emmett–Teller (BET) specific surface areas were found to be 688, 763 and 654 m2 g−1 for TBMID, TBMIDM and TBMIDE, respectively. All of the polymer networks showed high micropore surface area ratios over 90% and micropore volume ratios over 82% (Table 1), again suggesting that the pores of the polymer networks are dominated by micropores, which is in agreement with the shape of the nitrogen sorption isotherms.
 | ||
| Fig. 2 (a) Nitrogen adsorption (filled symbols)/desorption (empty symbols) isotherms for the polymer networks collected at 77.3 K; (b) pore size distribution curves calculated by NL-DFT. | ||
| Polymer | SBETa [m2 g−1] | SMicrob [m2 g−1] | VMicroc [cm3 g−1] | VTotald [cm3 g−1] | SMicro/SBET [%] | VMicro/VTotal [%] |
|---|---|---|---|---|---|---|
| a Surface area calculated from N2 adsorption isotherm in the relative pressure (P/Po) range from 0.05 to 0.20.b Micropore surface area calculated from the N2 adsorption isotherm using t-plot method based on the Harkins–Jura equation.c Micropore volume derived from the t-plot method.d Total pore volume at P/Po = 0.90. | ||||||
| TBMID | 688 | 629 | 0.30 | 0.35 | 91.4 | 85.7 |
| TBMIDM | 763 | 720 | 0.34 | 0.38 | 94.5 | 89.5 |
| TBMIDE | 654 | 588 | 0.28 | 0.34 | 89.9 | 82.4 |
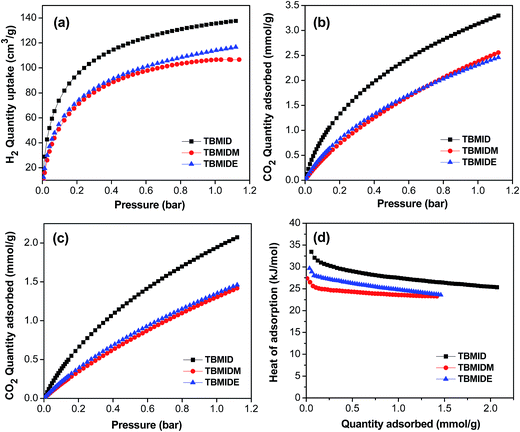
The microporous nature of these isoindigo functionalized polymer networks inspired us to investigate their gas uptake capacities. Fig. 3a shows the hydrogen sorption curves of the three polymer networks measured at 77.3 K up to a pressure of 1.13 bar. TBMID, without any alkyl substituents, exhibited the largest hydrogen uptake capacity of 137 cm3 g−1 (∼1.23 wt%) among the three polymer networks, although it showed a lower surface area of 688 m2 g−1 compared to that of TBMIDM (763 m2 g−1), which might be attributed to the narrower micropore size in TBMID enhancing the interaction between the pore wall and H2 molecules.48 The hydrogen uptake of TBMID is comparable to that of some other porous polymers with a much higher surface area under the same conditions, such as the dihydroxyl-functionalized conjugated microporous polymer of network-13 (1.14 wt%, SBET = 853 m2 g−1),49 the benzene-based CMP of CMP-0 (1.4 wt%, SBET = 1018 m2 g−1)50 and the nanoporous organic framework of NPOF-2 (1.45 wt%, SBET = 3127 m2 g−1),51 although this is still lower than that of the carbazole-based CMP, which has a hydrogen uptake capacity of 2.80 wt% at 1.13 bar/77.3 K.27
The CO2 uptakes of the polymer networks were measured up to 1.13 bar at 273 and 298 K, respectively (Fig. 3b and c). It was found that TBMID showed a high CO2 uptake ability of 3.30 mmol g−1 at 1.13 bar/273 K, which is higher than that of TBMIDM (2.56 mmol g−1) and TBMIDE (2.46 mmol g−1). The high CO2 uptake ability of TBMID could be attributed to the strong interactions between the polymer network and the polarizable CO2 molecules through dipole–quadrupole interactions and/or hydrogen bonding that utilizes the protonated and proton-free nitrogen sites of isoindigo segments,29 and the hydrogen bonding from the N–H of isoindigo segments might play a crucial role in increasing the CO2 uptake ability, since TBMIDM and TBMIDE showed a much lower CO2 uptake ability compared with TBMID, which could be attributed to the substitution of the hydrogen atom connected to the nitrogen atom with a methyl or ethyl group, which leads to a decreased hydrogen bonding interaction. A similar result was also observed for other types of porous polymers, for example, porous benzimidazole-linked polymers showed a high CO2 uptake ability of up to 5.3 mmol g−1 because the hydrogen atom connected with the nitrogen atom of the imidazole ring enhanced the interactions between the pore wall and CO2 molecules through hydrogen bonding.29 The CO2 uptake capacity of 3.30 mmol g−1 for TBMID is higher than that of many other reported CMPs produced by Sonogashira–Hagihara coupling reactions under the same conditions, such as the post-metalation of the porous aromatic framework of PAF-26-COOMg (2.85 mmol g−1, SBET = 572 m2 g−1),52 the amide-functionalized CMP of CMP-1-AMD1 (1.51 mmol g−1, SBET = 316 m2 g−1),47 the pyrene-based porous aromatic frameworks of PAF-20 (1.16 mmol g−1, SBET = 702 m2 g−1),53 the hexabenzocoronene-based porous organic polymers of HBC-POP-1 (2.05 mmol g−1 SBET = 668 m2 g−1),54 and the tri(4-ethynylphenyl)amine-based porous aromatic frameworks of PAF-34 (2.50 mmol g−1, SBET = 953 m2 g−1)55 at 273 K/1.0 bar. It is also much higher than that of some other types of porous materials with much higher BET surface areas under the same conditions, such as PAF-1 (2.1 mmol g−1, SBET = 5460 m2 g−1),56 COF-102 (1.56 mmol g−1, SBET = 3620 m2 g−1)57 and the tetraphenylmethane-based HCPs (1.66 mmol g−1, SBET = 1679 m2 g−1),58 although it is still low compared with some reported porous polymers with high carbon dioxide uptake capacity, such as an imine-linked porous polymer network (6.1 mmol g−1 for PPF-1),28 fluorinated covalent triazine-based frameworks (5.53 mmol g−1 for FCTF-1-600),59 carbazolic porous organic frameworks (4.77 mmol g−1 for Cz-POF-3),60 and benzimidazole-linked polymers (5.3 mmol g−1 for BILP-4).29 It is known that surface area, micropore volume, pore size and polar groups or moieties have large influences on the CO2 adsorption performance of microporous organic polymers. For example, TBMID, with a smaller pore size of 0.9 nm, showed a higher CO2 uptake ability of 3.3 mmol g−1 than TBMIDM (pore size 1.1 nm) and TBMIDE (pore size 1.0 nm), which could be attributed to the stronger binding affinity between TBMID and CO2 molecules, as evidenced by the isosteric heat of CO2 adsorption (Fig. 3d). Therefore, one could expect that the CO2 uptake ability of these isoindigo-based microporous organic polymers could be further improved by optimizing the surface area, pore size or introducing some strong polar groups into the skeleton of the polymers.
The isosteric heat of adsorption (Qst) was calculated from the CO2 adsorption data collected at 273 K and 298 K by the Clausius–Clapeyron equation to determine the binding affinity between the polymers and CO2 molecules. As shown in Fig. 3d, the Qst values of the polymer networks were found to be in the range of 27.4–33.5 kJ mol−1 at near zero-coverage, which are comparable to those of many known functionalized porous polymers, such as porous benzimidazole-linked polymers (26.7–28.8 kJ mol−1),29 carbazole-based CMPs (27.1–30.8 kJ mol−1),43 tetraphenylethylene-based HCPs (23.3–28.2 kJ mol−1),61 the metalation of CMPs (PAF-26-COONa, 35 kJ mol−1),52 sulfonated porous polymer networks (PPN-6-SO3Li, 35.7 kJ mol−1)62 and porous polymer networks (PPN-6-SO3NH4, 40 kJ mol−1).63 This result demonstrated that the incorporation of the isoindigo unit into the skeleton of the porous polymers indeed enhanced the binding affinity between the porous polymer and CO2 molecules, thus leading to the increased CO2 uptake ability. In addition, TBMID showed the highest Qst (33.5 kJ mol−1) among the three polymer networks owing to its smaller micropore size and the strong interactions between the polymer network and the polarizable CO2 molecules through dipole–quadrupole interactions and/or hydrogen bonding, as discussed above. In order to investigate the potential use of these isoindigo-functionalized microporous organic polymers in gas separation, single component gas adsorption isotherms for CO2, N2, and CH4 were measured by volumetric methods at 273 K up to a pressure of 1.0 bar. The selectivities of CO2/N2 and CO2/CH4 for the polymer networks were estimated by using the initial slope ratios from the Henry’s law constants of the single-component gas adsorption isotherms collected at 273 K at a low pressure coverage of less than 0.1 bar, as summarized in Table 2 (Fig. S7–9†). Compared with TBMIDM and TBMIDE, TBMID showed higher CO2/N2 and CO2/CH4 adsorption selectivities (Table 2). Though the CO2/N2 adsorption selectivity of 58.8 for TBMID is moderate compared with those of some other reported MOPs with higher CO2/N2 adsorption selectivities, such as the tetraphenylethylene-based HCPs (119 for Network-7),61 azo-bridged covalent organic polymer (109 for Azo-COP-2),64 porous covalent electron-rich organonitridic frameworks (109 for PECONF-1),65 and tri(4-ethynylphenyl) amine-based porous aromatic frameworks (251 for PAF-33-NH2),55 TBMID showed much higher CO2 adsorption capacity (Network-7: 1.92 mmol g−1,61 Azo-COP-2: 2.55 mmol g−1,64 PECONF-1: 1.86 mmol g−1,65 and PAF-33-NH2: 1.19 mmol g−1 (ref. 55)) under the same conditions. From a practical CO2 separation application of view, both high CO2 capture capacity and high selectivity of CO2 over N2 and CH4 are required. Therefore, these isoindigo-based microporous organic polymers could be potential candidates for applications in post-combustion CO2 capture and sequestration because of their good CO2 adsorption performances.
| Polymer | H2 uptakea [wt%] | CH4 uptakeb [mmol g−1] | CO2 uptakeb [mmol g−1] | CO2 uptakec [mmol g−1] | Selectivityd | |
|---|---|---|---|---|---|---|
| CO2/N2 | CO2/CH4 | |||||
| a Data obtained at 77.3 K and 1.13 bar.b Data collected at 273 K and 1.13 bar.c Data collected at 298 K and 1.13 bar.d Adsorption selectivity based on Henry’s law. | ||||||
| TBMID | 1.23 | 0.75 | 3.30 | 2.07 | 58.8 | 9.7 |
| TBMIDM | 0.95 | 0.79 | 2.56 | 1.42 | 29.3 | 4.5 |
| TBMIDE | 1.04 | 0.86 | 2.46 | 1.46 | 42.6 | 4.3 |
4. Conclusions
In conclusion, isoindigo functionalized microporous organic polymers have been synthesized via a palladium-catalyzed Sonogashira–Hagihara cross-coupling reaction of 6,6′-dibromoisoindigo, or its alkylated derivatives, with tetra(4-ethynylphenyl)-methane. These polymer networks are stable in various solvents that were tested and thermally stable in a nitrogen atmosphere. The alkyl groups have an influence on the pore size and surface area of the resulting microporous organic polymers. Owing to the incorporation of the electron-rich isoindigo unit into the skeleton of the porous polymers, which enhanced the binding affinity between the porous polymer and CO2 molecules, all of the resulting polymers show high isosteric heats of CO2 adsorption from 27.4 to 33.5 kJ mol−1. The polymer TBMID, without any alkyl substituent, shows a high CO2 uptake ability of 3.30 mmol g−1 and a CO2/N2 adsorption selectivity of 58.8 because of the strong interactions between the polymer network and the polarizable CO2 molecules through dipole–quadrupole interactions and/or hydrogen bonding that utilize the protonated and proton-free nitrogen sites of the isoindigo segments. These results indicated that there is a wealth of opportunity for producing CO2-philic microporous organic polymers with enhanced gas adsorption abilities and high adsorption selectivities towards CO2 over N2 by incorporating an electron-rich unit into the skeleton of a MOP.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21304055), Research Fund for the Doctoral Program of Higher Education of China (20120202120007), the Shaanxi Innovative Team of Key Science and Technology (2013KCT-17), the Fundamental Research Funds for the Central Universities (GK201501002, GK201101003 & GK201301002), and the Open Fund of the State Key Laboratory of Luminescent Materials and Devices (2015-skllmd-04).References
- F. X. Li and L. S. Fan, Energy Environ. Sci., 2008, 1, 248–267 Search PubMed.
- R. V. Siriwardane, M. S. Shen and E. P. Fisher, Energy Fuels, 2003, 17, 571–576 CrossRef CAS.
- C. Lu, H. Bai, B. Wu, F. S. Su and J. F. Hwang, Energy Fuels, 2008, 22, 3050–3056 CrossRef CAS.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2011, 112, 724–781 CrossRef PubMed.
- T. Tozawa, J. T. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Slawin, A. Steiner and A. I. Cooper, Nat. Mater., 2009, 8, 973–978 CrossRef CAS PubMed.
- Y. Jin, B. A. Voss, A. Jin, H. Long, R. D. Noble and W. Zhang, J. Am. Chem. Soc., 2011, 133, 6650–6658 CrossRef CAS PubMed.
- R. Dawson, A. I. Cooper and D. J. Adams, Prog. Polym. Sci., 2012, 37, 530–563 CrossRef CAS.
- Z. H. Xiang and D. Cao, J. Mater. Chem. A, 2013, 1, 2691–2718 RSC.
- N. B. McKeown and P. M. Budd, Macromolecules, 2010, 43, 5163–5176 CrossRef CAS.
- T. C. Drage, C. E. Snape, L. A. Stevens, J. Wood, J. Wang, A. I. Cooper, R. Dawson, X. Guo, C. Satterley and R. Irons, J. Mater. Chem., 2012, 22, 2815–2823 RSC.
- Y. Zhang and S. N. Riduan, Chem. Soc. Rev., 2012, 41, 2083–2094 RSC.
- P. Kaur, J. T. Hupp and S. T. Nguyen, ACS Catal., 2011, 1, 819–835 CrossRef CAS.
- X. M. Liu, Y. H. Xu and D. L. Jiang, J. Am. Chem. Soc., 2012, 134, 8738–8741 CrossRef CAS PubMed.
- Z. H. Xiang and D. P. Cao, Macromol. Rapid Commun., 2012, 33, 1184–1190 CrossRef CAS PubMed.
- P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Chem. Commun., 2004, 230–231 RSC.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O’Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- J. X. Jiang, F. Su, A. Trewin, C. D. Wood, N. L. Campbell, H. Niu, C. Dickinson, A. Y. Ganin, M. J. Rosseinsky, Y. Z. Khimyak and A. I. Cooper, Angew. Chem., Int. Ed, 2007, 46, 8574–8578 CrossRef CAS PubMed.
- A. I. Cooper, Adv. Mater., 2009, 21, 1291–1295 CrossRef CAS.
- J. X. Jiang and A. I. Cooper, Top. Curr. Chem., 2010, 293, 1–33 CAS.
- D. Yuan, W. Lu, D. Zhao and H. C. Zhou, Adv. Mater., 2011, 23, 3723–3725 CrossRef CAS PubMed.
- T. Ben, H. Ren, S. Ma, D. Cao, J. Lan, X. Jing, W. Wang, J. Xu, F. Deng, J. M. Simmons, S. Qiu and G. Zhu, Angew. Chem., Int. Ed, 2009, 48, 9457–9460 CrossRef CAS PubMed.
- P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed, 2008, 47, 3450–3453 CrossRef CAS PubMed.
- J. Germain, J. Hradil, J. M. Fréchet and F. Svec, Chem. Mater., 2006, 18, 4430–4435 CrossRef CAS.
- J. Y. Lee, C. D. Wood, D. Bradshaw, M. J. Rosseinsky and A. I. Cooper, Chem. Commun., 2006, 2670–2672 RSC.
- S. J. Xu, Y. L. Luo and B. Tan, Macromol. Rapid Commun., 2013, 34, 471–484 CrossRef CAS PubMed.
- Q. Chen, M. Luo, P. Hammershoj, D. Zhou, Y. Han, B. W. Laursen, C. G. Yan and B. H. Han, J. Am. Chem. Soc., 2012, 134, 6084–6087 CrossRef CAS PubMed.
- Y. L. Zhu, H. Long and W. Zhang, Chem. Mater., 2013, 25, 1630–1635 CrossRef CAS.
- M. G. Rabbani and H. M. El-Kaderi, Chem. Mater., 2012, 24, 1511–1517 CrossRef CAS.
- P. Arab, M. G. Rabbani, A. K. Sekizkardes, T. İslamoğlu and H. M. El-Kaderi, Chem. Mater., 2014, 26, 1385–1392 CrossRef CAS.
- X. Zhu, S. M. Mahurin, S. H. An, C. L. Do-Thanh, C. Tian, Y. Li, L. W. Gill, E. W. Hagaman, Z. Bian, J. H. Zhou, J. Hu, H. Liu and S. Dai, Chem. Commun., 2014, 50, 7933–7936 RSC.
- J. Byun, S.-H. Je, H. A. Patel, A. Coskun and C. T. Yavuz, J. Mater. Chem. A, 2014, 2, 12507–12512 RSC.
- A. Modak, M. Nandi, J. Mondal and A. Bhaumik, Chem. Commun., 2012, 48, 248–250 RSC.
- A. Modak, M. Pramanik, S. Inagaki and A. Bhaumik, J. Mater. Chem. A, 2014, 2, 11642–11650 RSC.
- R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1173–1177 RSC.
- J. X. Jiang, C. Wang, A. Laybourn, T. Hasell, R. Clowes, Y. Z. Khimyak, J. Xiao, S. J. Higgins, D. J. Adams and A. I. Cooper, Angew. Chem., Int. Ed, 2011, 50, 1072–1075 CrossRef CAS PubMed.
- P. Zhang, Z. Weng, J. Guo and C. Wang, Chem. Mater., 2011, 23, 5243–5249 CrossRef CAS.
- Y. Xie, T. T. Wang, X. H. Liu, K. Zou and W. Q. Deng, Nat. Commun., 2013, 4, 1960 Search PubMed.
- R. Stalder, J. Mei, K. R. Graham, L. A. Estrada and J. R. Reynolds, Chem. Mater., 2013, 26, 664–678 CrossRef.
- W. Ying, F. Guo, J. Li, Q. Zhang, W. Wu, H. Tian and J. Hua, ACS Appl. Mater. Interfaces, 2012, 4, 4215–4224 Search PubMed.
- S. W. Yuan, S. Kirklin, B. Dorney, D. J. Liu and L. P. Yu, Macromolecules, 2009, 42, 1554–1559 CrossRef CAS.
- K. Sing, D. Everett, R. Haul, L. Moscou, R. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- X. Y. Wang, Y. Zhao, L. L. Wei, C. Zhang, X. Yang, M. Yu and J. X. Jiang, Macromol. Chem. Phys., 2015, 216, 504–510 CrossRef CAS.
- R. Banerjee, H. Furukawa, D. Britt, C. Knobler, M. O’Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 3875–3877 CrossRef CAS PubMed.
- B. P. Biswal, S. Chandra, S. Kandambeth, B. Lukose, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 5328–5331 CrossRef CAS PubMed.
- L. M. Lanni, R. W. Tilford, M. Bharathy and J. J. Lavigne, J. Am. Chem. Soc., 2011, 133, 13975–13983 CrossRef CAS PubMed.
- T. Ratvijitvech, R. Dawson, A. Laybourn, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Polymer, 2014, 55, 321–325 CrossRef CAS.
- C. Zhang, X. Yang, Y. Zhao, X. Wang, M. Yu and J. X. Jiang, Polymer, 2015, 61, 36–41 CrossRef CAS.
- R. Dawson, A. Laybourn, R. Clowes, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Macromolecules, 2009, 42, 8809–8816 CrossRef CAS.
- J. X. Jiang, F. Su, A. Trewin, C. D. Wood, H. Niu, J. T. Jones, Y. Z. Khimyak and A. I. Cooper, J. Am. Chem. Soc., 2008, 130, 7710–7720 CrossRef CAS PubMed.
- R. M. Kassab, K. T. Jackson, O. M. El-Kadri and H. M. El-Kaderi, Res. Chem. Intermed., 2011, 37, 747–757 CrossRef CAS.
- H. P. Ma, H. Ren, X. Q. Zou, S. Meng, F. X. Sun and G. S. Zhu, Polym. Chem., 2014, 5, 144–152 RSC.
- Z. J. Yan, H. Ren, H. P. Ma, R. R. Yuan, Y. Yuan, X. Q. Zou, F. X. Sun and G. S. Zhu, Microporous Mesoporous Mater., 2013, 173, 92–98 CrossRef CAS.
- C. M. Thompson, F. Li and R. A. Smaldone, Chem. Commun., 2014, 50, 6171–6173 RSC.
- R. R. Yuan, H. Ren, Z. J. Yan, A. Wang and G. S. Zhu, Polym. Chem., 2014, 5, 2266 RSC.
- T. Ben, C. Y. Pei, D. L. Zhang, J. Xu, F. Deng, X. F. Jing and S. L. Qiu, Energy Environ. Sci., 2011, 4, 3991 Search PubMed.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS PubMed.
- X. F. Jing, D. L. Zou, P. Cui, H. Ren and G. S. Zhu, J. Mater. Chem. A, 2013, 1, 13926–13931 RSC.
- Y. F. Zhao, K. X. Yao, B. Y. Teng, T. Zhang and Y. Han, Energy Environ. Sci., 2013, 6, 3684–3692 Search PubMed.
- X. Zhang, J. Z. Lu and J. Zhang, Chem. Mater., 2014, 26, 4023–4029 CrossRef CAS.
- S. W. Yao, X. Yang, M. Yu, Y. H. Zhang and J. X. Jiang, J. Mater. Chem. A, 2014, 2, 8054–8059 RSC.
- W. Lu, D. Yuan, J. Sculley, D. Zhao, R. Krishna and H. C. Zhou, J. Am. Chem. Soc., 2011, 133, 18126–18129 CrossRef CAS PubMed.
- W. G. Lu, W. M. Verdegaal, J. M. Yu, P. B. Balbuena, H.-K. Jeong and H.-C. Zhou, Energy Environ. Sci., 2013, 6, 3559–3564 Search PubMed.
- H. A. Patel, S. H. Je, J. Park, Y. Jung, A. Coskun and C. T. Yavuz, Chem.–Eur. J., 2014, 20, 772–780 CrossRef CAS PubMed.
- P. Mohanty, L. D. Kull and K. Landskron, Nat. Commun., 2011, 2, 401 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the synthesis for TBMIDM, TBMIDE and the TGA, FT-IR, PXRD, SEM, HR-TEM images, and gas adsorption for the polymer networks. See DOI: 10.1039/c5ra19226a |
| This journal is © The Royal Society of Chemistry 2015 |