Gram-scale synthesis of high-purity graphene quantum dots with multicolor photoluminescence†
Fuchi Liuab,
Yuanyuan Suna,
Yongping Zhenga,
Nujiang Tang*a,
Ming Lia,
Wei Zhonga and
Youwei Dua
aNanjing National Laboratory of Microstructures & Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing 210093, People’s Republic of China. E-mail: tangnujiang@nju.edu.cn
bCollege of Physics and Technology, Guangxi Normal University, Guilin 541004, People’s Republic of China
First published on 24th November 2015
Abstract
A gram-scale approach has been developed to prepare highly pure graphene quantum dots (GQDs) from Vulcan XC-72 carbon black refluxed with concentrated nitric acid using a home-built experimental system. The weight of the GQDs is high, up to 1.2 g in each run with a yield of 75 wt%, and the purity is 99.96 wt%. The results show that the GQDs exhibit multicolor photoluminescence from green to light red under different excitation wavelengths.
Graphene quantum dots (GQDs) have aroused tremendous interest in nanoscience and nanotechnology due to their unique properties, such as their large surface area, low cytotoxicity, excellent solubility, tunable bandgap,1,2 quantum confinement and edge effects,3,4 etc. GQDs are emerging as promising nanomaterials for prospective applications in biological imaging and labeling,5–9 ultrasensitive sensors and detection,10–16 photoluminescent probes,17 supercapacitors,18–20 and solar cells,21–23 etc. It’s worth noting that for biological applications of carbon nanomaterials in imaging receptors on living cells,24 molecular diagnostic systems and nanotechnological research,25 etc. high purity is essential.
Recently, a variety of methods have been reported to synthesize GQDs, including microwave,2 hydrothermal,4 electrochemical,26 and high-resolution electron-beam27 approaches, and so on. Generally, these methods suffered from the use of expensive materials such as graphene oxide (GO), reduced GO, or carbon fibre as the precursor, and the yields of the GQDs obtained are very low (<5 wt%), which limits the spread and application of the GQDs.28 Moreover, some effort has been taken to develop new methods for the high-yield synthesis of GQDs in recent years. For example, Sun et al. synthesized GQDs by the oxidation of natural graphite powder.28 Shin et al. reported the one-pot synthesis of GQDs by the high-powered microwave irradiation of graphite.1 Liu et al. synthesized gram-scale GQDs from active carbon atoms using the Hummer’s method.29 Wang et al. reported a gram-scale synthesis of single-crystalline GQDs by a molecular fusion route under hydrothermal conditions, and the synthesis involves the nitration of pyrene followed by hydrothermal treatment in alkaline aqueous solutions.30 However, the purity of the GQDs is low. Thus, the purification of the GQDs obtained is needed, and which typically requires a very long time to ensure acid or alkali removal. More importantly, purification always involves a process that requires a strong base or acid, resulting in the introduction of a large amount of salt in the GQDs. To obtain high-purity GQDs, Dong et al. reported the synthesis of GQDs from carbon black refluxed with concentrated nitric acid without using any other reagent.31 Therein, the yield of the GQDs is still low. Thus, it is of great significance to develop a method for the synthesis of GQDs with both high yield and high purity.
Additionally, multicolor fluorescent nanomaterials have aroused continual interest. Typically, considerable studies have focused on enhancing the multicolor photoluminescence (PL) or photodetection of fluorescent materials by means of graphene-based materials. For example, Zhu et al. developed a new strategy for multiple oligonucleotide target detection with three dye-labeled nucleic acid probes and GO.32 Zhou et al. successfully designed an effective theranostic platform with multicolor imaging and satisfactory photo-induced anticancer activity based on porous silica nanoparticles encapsulated with the complex of a photodynamic anticancer drug and GQDs.33 Notably, little work has been done on the multicolor optical properties of GQDs.
Herein, we report a gram-scale synthesis of highly pure GQDs from Vulcan XC-72 carbon black (Cabot Corporation). The results show that 1.2 g of GQDs with a high yield of 75 wt% and a high purity of 99.96 wt% can be obtained in each run. More interestingly, the GQDs show multicolor PL from green to light red.
As illustrated in Fig. 1a, the GQDs were prepared using a modified version of Dong’s method31 using a home-built experimental system. The main changes are shown below: (i) 15 mol L−1 HNO3 was used to increase the oxidation capacity; (ii) ethylene glycol was used to replace water as the coolant and was cooled at −5 °C by a low-temperature constant temperature bath to increase the reflux efficiency; (iii) 220 and 25 nm microporous membranes were used successively to remove unoxidized carbon black or big particles; (iv) the integrative home-built experimental system ensures the oxidation of carbon black and the evaporation of nitric acid can be finished under in situ and eco-friendly conditions. Fig. 1b shows the schematic illustrations of the mechanism for GQD formation from spherical carbon black. In a typical procedure, 1.6 g of dried XC-72 carbon black and 300 mL of 15 mol L−1 HNO3 were put into a 1000 mL round bottom flask. The switches 1, 2, and 3 were opened and switches 4 and 5 were shut. Then, the mixture was heated in an oil bath at 135 °C followed by refluxing and magnetic stirring for 24 h. At the same time, a condenser tube was cooling at −5 °C using cryogenic ethylene glycol. To prevent the splattering of the hot oil, a specially designed water withdrawal device was used to remove the condensate water attached to the condenser tube. After refluxing for 24 h, switches 1, 2, and 3 were turned off, and switches 4 and 5 were turned on. The mixture was heated at 180 °C for approximately 10 h under a flowing Ar atmosphere (60 mL min−1) to evaporate the concentrated nitric acid, and a small pile of light black solid was obtained. Thereafter, the solid was redissolved in 2 L of deionized water (Fig. S1a†), and then the solution was centrifuged (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) for 30 min to remove unoxidized carbon black or big particles. The obtained supernatant was vacuum filtered using 220 and 25 nm microporous membranes successively, and unoxidized carbon black or the big particles were collected on the membranes (Fig. S1b and c†). Subsequently, the obtained reddish-brown GQD solution (Fig. S1d†) was evaporated using rotary evaporators at 80 °C. After the obtained, approximately 200 mL, concentrated GQD solution was dried by a vacuum freeze drier, 1.2 g of claybank powdered GQDs (Fig. 1c) were obtained. Note that, the weight is 13.5 times higher than that of reported GQDs (ca. 0.089 g).31
000 rpm) for 30 min to remove unoxidized carbon black or big particles. The obtained supernatant was vacuum filtered using 220 and 25 nm microporous membranes successively, and unoxidized carbon black or the big particles were collected on the membranes (Fig. S1b and c†). Subsequently, the obtained reddish-brown GQD solution (Fig. S1d†) was evaporated using rotary evaporators at 80 °C. After the obtained, approximately 200 mL, concentrated GQD solution was dried by a vacuum freeze drier, 1.2 g of claybank powdered GQDs (Fig. 1c) were obtained. Note that, the weight is 13.5 times higher than that of reported GQDs (ca. 0.089 g).31
Fig. 2a and b show the typical transmission electron microscopy (TEM) images and diameter distributions of the GQDs. It is found that GQDs are relatively uniform with diameters of about 2–6 nm. According to the calculations, one can find that the average diameter of the GQDs is ca. 4.2 nm (Fig. 2b), obviously smaller than that of those previous reported (ca. 15 nm).31 The inset of Fig. 2a shows the HR-TEM image of a GQD, which shows that it has a discernible lattice structure. Fig. 2c, d and e present the atomic force microscope (AFM) image of the GQDs, their height distribution, and the height line profile, respectively. One can see that the average height of the GQDs is ca. 1.05 nm, and more than 89% of the GQDs are less than 2 nm in height. This demonstrates that the obtained GQDs primarily consist of 1–3 layers.26,34
 | ||
| Fig. 2 Microstructure of the as-prepared GQDs, (a) TEM image. Inset of (a): HR-TEM image. (b) Diameter distribution. (c) AFM image. (d) Height distribution. (e) Height profile along the line A–B. | ||
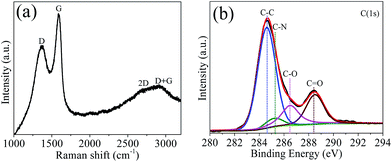
Fig. 3a shows the Raman spectrum of the GQDs. One can see that there are two prominent peaks of the D and G band at 1366 and 1590 cm−1, respectively. It is known that the D band is a disordered band arising from the disorder in sp2 hybridized carbon, while the G band corresponds to the first-order scattering of the stretching vibration mode E2g observed for sp2 carbon domains.35 The broad bands centered at 2680 and 2935 cm−1 correspond to the 2D and D + G bands, respectively.36 They arise from relaxation in the selection rules caused by phonon scattering at boundaries and defects in the GQDs.35,37 Based on the Raman spectrum, one can find that the intensity ratio of the D band to the G band (ID/IG) is about 0.81. As reported by Tuinstra and Koenig,38 ID/IG varies inversely with the cluster size La in nanocrystalline graphite according to this relationship: ID/IG = C(λ)/La, where C(λ) is an empirical constant that depends on the excitation laser energy and C (λ = 515.5 nm) = 4.4 nm.38,39 According to this relation, the average size of the GQDs is La = 5.4 nm, which falls within the 2–6 nm range obtained using the TEM data described above.
To determine the composition of the GQDs, an X-ray photoemission spectroscopy (XPS) measurement was employed. Based on the XPS results (Fig. 3b), it is found that the GQDs are oxygen-rich with a high oxygen content defined as 100 O/C at% of ca. 53.7 at%. The high-resolution C 1s peaks of the GQDs can be deconvoluted into several peaks corresponding to the C–C bond in aromatic rings (284.6 eV), C–O bond (286.3 eV), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (288.4 eV).26 In addition, a little peak corresponding to the C–N bond (285.2 eV) also can be detected.
O bond (288.4 eV).26 In addition, a little peak corresponding to the C–N bond (285.2 eV) also can be detected.
To evaluate the purity of the obtained GQDs, alternative GQDs were prepared using the universal hydrothermal method4 from GO sheets, which were named as GQDs-1. Table 1 shows the impurity content of the GQDs and GQDs-1. One can see that the impurity content (such as Fe, Ni, Mn, and Cu) of the GQDs is much lower than that of the GQDs-1. In particular, the content of the Na and Ca elements, which may inevitably be introduced from deionized water used during the preparation process, is only 130.7 and 270.5 ppm for the GQDs, respectively. By contrast, in the case of the GQDs-1, it is high, up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 and 44
330 and 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 ppm, which is ca. 80 and 163 times higher than that of the GQDs. The reasons for this may be that (i) the synthesis of the GQDs-1 involves a neutralization process which requires a strong base; and (ii) the concentration process for concentrating much of the water solution after dialysis, results in the introduction of a large amount of salt. Clearly, all the results indicate that the purity of our GQDs is much higher than that of the GQDs prepared using the universal hydrothermal method. Namely, we have synthesized GQDs with high yield and purity through one-step chemical oxidation using our home-built experimental system.
030 ppm, which is ca. 80 and 163 times higher than that of the GQDs. The reasons for this may be that (i) the synthesis of the GQDs-1 involves a neutralization process which requires a strong base; and (ii) the concentration process for concentrating much of the water solution after dialysis, results in the introduction of a large amount of salt. Clearly, all the results indicate that the purity of our GQDs is much higher than that of the GQDs prepared using the universal hydrothermal method. Namely, we have synthesized GQDs with high yield and purity through one-step chemical oxidation using our home-built experimental system.
| Samples (ppm) | Fe | Ni | Mn | Cu | Na | Ca |
|---|---|---|---|---|---|---|
| GQDs | 2.6 | 0.8 | 0.8 | 3.6 | 130.7 | 270.5 |
| GQDs-1 | 96.3 | 11.1 | 38.3 | 18.5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 330 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
For spectral investigation, 0.05 mg per 1 mL of sample solution was made by ultrasonically dispersing the solid GQDs in distilled water. As shown in Fig. 4a, the GQDs show a broad UV-vis absorption below 600 nm with one peak at ca. 200 nm, and with three shoulder peaks at ca. 225, 300, and 440 nm, respectively. Clearly, it is very different from that of the GQDs previously reported.31 Considering the fact that the size of our GQDs is obviously smaller than that of those previously reported, the size effect may be responsible for the observed difference of the UV-vis absorption spectrum.
Fig. 4b shows the detailed PL of the GQDs. One can find that the GQDs exhibit an excitation-independent PL behavior with an emission peak position at 523 nm when the excitation wavelengths changed from 260 to 470 nm. By contrast, when the excitation wavelength changed from 470 to 580 nm, the maximum emission peak position shifted from 523 to 620 nm with a red-shift of about 100 nm, showing clear excitation wavelength dependence. In brief, the GQDs have preeminent multicolor fluorescence emission depending on different excitations. Furthermore, the full width at half maximum (FWHM) at the excitation of 470 nm is only 110 nm, further confirming the narrow size distribution of the as-prepared GQDs. The multicolor photoluminescence feature of the GQDs was also observed under different excitation wavelengths through a fluorophotometer. As shown in the inset of Fig. 4a, the solution of the GQDs exhibits green, yellow green, yellow, orange and light red PL, under ultraviolet to blue (260–500 nm), green (510–530 nm), dark green (540–550 nm), yellow green (555–570 nm) and yellow (580–595 nm) light excitation, respectively. This should be noted that as the excitation wavelength red-shifted, the emission wavelength could reach light red regions. It suggests that our GQDs can be used in cell imaging.40 The origins of PL in the GQDs are not clear so far. One can propose that this observed multicolor PL may be attributed to the different distribution of emissive traps on the surface of the GQDs, as suggested in the literature.41,42
The quantum yield (QY) of the GQDs was assumed by using quinine sulfate as a reference (Table 2). It was calculated according to
| ϕ = ϕr(I/Ir)(n2/nr2)(Ar/A) |
| Sample | Integrated emission intensity (I) | Abs. (A) | Refractive index of solvent (n) | Quantum yield (ϕ) |
|---|---|---|---|---|
| Quinine sulfate | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 134.5 134.5 |
0.0523 | 1.33 | 0.54 (known) |
| GQDs | 4321.6 | 0.0371 | 1.33 | 0.051 |
Conclusions
We reported a one-step pathway for the gram-scale synthesis of high-purity GQDs by refluxing Vulcan XC-72 carbon black with concentrated nitric acid using a home-built experimental system. The results showed that the yield of the GQDs is high, up to 1.2 g in each run by using 1.6 g of Vulcan XC-72 carbon black as the starting material, and the purity is 99.96 wt%. It is found that the GQDs show evident multicolor PL features. All the results suggest that the high-purity GQDs with multicolor PL have great potential for optoelectronic and biomedical applications.Acknowledgements
This work was financially supported by the State Key Program for Basic Research (Grant No. 2014CB921102 and 2012CB932304), NSFC (Grant No. U1232210 and 11164003), Guangxi Natural Science Foundation (Grant No. 2015GXNSFAA139015), Foundation of Guangxi Educational Committee (Grant No. KY2015YB036), and Key Programs of Guangxi Normal University (Grant No. 2014ZD003), P. R. China.Notes and references
- Y. Shin, J. Lee, J. Yang, J. Park, K. Lee, S. Kim, Y. Park and H. Lee, Small, 2014, 10, 866–870 CrossRef CAS PubMed
.
- L. L. Li, J. Ji, R. Fei, C. Z. Wang, Q. Lu, J. R. Zhang, L. P. Jiang and J. J. Zhu, Adv. Funct. Mater., 2012, 22, 2971–2979 CrossRef CAS
.
- X. Yan, X. Cui, B. Li and L. s. Li, Nano Lett., 2010, 10, 1869–1873 CrossRef CAS PubMed
.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734–738 CrossRef CAS PubMed
.
- H. Z. Pan, M. Xu, L. Chen, Y. Y. Sun and Y. L. Wang, Acta Biochim. Biophys. Sin., 2010, 59, 6443–6449 CAS
.
- Y. Wang, L. Zhang, R. P. Liang, J. M. Bai and J. D. Qiu, Anal. Chem., 2013, 85, 9148–9155 CrossRef CAS PubMed
.
- S. Zhu, J. Zhang, C. Qiao, S. Tang, Y. Li, W. Yuan, B. Li, L. Tian, F. Liu, R. Hu, H. Gao, H. Wei, H. Zhang, H. Sun and B. Yang, Chem. Commun., 2011, 47, 6858–6860 RSC
.
- S. Chen, X. Hai, C. Xia, X. W. Chen and J. H. Wang, Chem.–Eur. J., 2013, 19, 15918–15923 CrossRef CAS PubMed
.
- X. T. Zheng, H. L. He and C. M. Li, RSC Adv., 2013, 3, 24853–24857 RSC
.
- J. J. Lu, M. Yan, L. Ge, S. G. Ge, S. W. Wang, J. X. Yan and J. H. Yu, Biosens. Bioelectron., 2013, 47, 271–277 CrossRef CAS PubMed
.
- Z. S. Qian, X. Y. Shan, L. J. Chai, J. J. Ma, J. R. Chen and H. Feng, Biosens. Bioelectron., 2014, 60, 64–70 CrossRef CAS PubMed
.
- H. J. Sun, N. Gao, L. Wu, J. S. Ren, W. L. Wei and X. G. Qu, Chem.–Eur. J., 2013, 19, 13362–13368 CrossRef CAS PubMed
.
- C. Zhou, W. Jiang and B. K. Via, Colloids Surf., B, 2014, 118, 72–76 CrossRef CAS PubMed
.
- H. Sun, L. Wu, W. Wei and X. Qu, Mater. Today, 2013, 16, 433–442 CrossRef CAS
.
- J. P. Tian, H. M. Zhao, X. Quan, Y. B. Zhang, H. T. Yu and S. Chen, Sens. Actuators, B, 2014, 196, 532–538 CrossRef CAS
.
- F. X. Wang, Z. Y. Gu, W. Lei, W. J. Wang, X. F. Xia and Q. L. Hao, Sens. Actuators, B, 2014, 190, 516–522 CrossRef CAS
.
- J. M. Bai, L. Zhang, R. P. Liang and J. D. Qiu, Chem.–Eur. J., 2013, 19, 3822–3826 CrossRef CAS PubMed
.
- Y. Hu, Y. Zhao, G. Lu, N. Chen, Z. Zhang, H. Li, H. Shao and L. Qu, Nanotechnology, 2013, 24, 1–7 Search PubMed
.
- W. Liu, X. Yan, J. Chen, Y. Feng and Q. Xue, Nanoscale, 2013, 5, 6053–6062 RSC
.
- W. W. Liu, Y. Q. Feng, X. B. Yan, J. T. Chen and Q. J. Xue, Adv. Funct. Mater., 2013, 23, 4111–4122 CrossRef CAS
.
- J. K. Kim, M. J. Park, S. J. Kim, D. H. Wang, S. P. Cho, S. Bae, J. H. Park and B. H. Hong, ACS Nano, 2013, 7, 7207–7212 CrossRef CAS PubMed
.
- Z. L. Zhu, J. A. Ma, Z. L. Wang, C. Mu, Z. T. Fan, L. L. Du, Y. Bai, L. Z. Fan, H. Yan, D. L. Phillips and S. H. Yang, J. Am. Chem. Soc., 2014, 136, 3760–3763 CrossRef CAS PubMed
.
- H. B. Yang, Y. Q. Dong, X. Z. Wang, S. Y. Khoo, B. Liu and C. M. Li, Sol. Energy Mater. Sol. Cells, 2013, 117, 214–218 CrossRef CAS
.
- M. Howarth, W. Liu, S. Puthenveetil, Y. Zheng, L. F. Marshall, M. M. Schmidt, K. D. Wittrup, M. G. Bawendi and A. Y. Ting, Nat. Methods, 2008, 5, 397–399 CrossRef CAS PubMed
.
- P. M. E. Gramlich, C. T. Wirges, A. Manetto and T. Carell, Angew. Chem., Int. Ed., 2008, 47, 8350–8358 CrossRef CAS PubMed
.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776–780 CrossRef CAS PubMed
.
- L. A. Ponomarenko, F. Schedin, M. I. Katsnelson, R. Yang, E. W. Hill, K. S. Novoselov and A. K. Geim, Science, 2008, 320, 356–358 CrossRef CAS PubMed
.
- Y. Sun, S. Wang, C. Li, P. Luo, L. Tao, Y. Wei and G. Shi, Phys. Chem. Chem. Phys., 2013, 15, 9907–9913 RSC
.
- Y. Liu, B. Gao, Z. Qiao, Y. Hu, W. Zheng, L. Zhang, Y. Zhou, G. Ji and G. Yang, Chem. Mater., 2015, 27, 4319–4327 CrossRef CAS
.
- L. Wang, Y. Wang, T. Xu, H. Liao, C. Yao, Y. Liu, Z. Li, Z. Chen, D. Pan, L. Sun and M. Wu, Nat. Commun., 2014, 5, 5357 CrossRef CAS PubMed
.
- Y. Dong, C. Chen, X. Zheng, L. Gao, Z. Cui, H. Yang, C. Guo, Y. Chi and C. M. Li, J. Mater. Chem., 2012, 22, 8764–8766 RSC
.
- Q. Zhu, D. Xiang, C. Zhang, X. Ji and Z. He, Analyst, 2013, 138, 5194–5196 RSC
.
- L. Zhou, X. Ge, J. Zhou, S. Wei and J. Shen, Chem. Commun., 2015, 51, 421–424 RSC
.
- X. Li, X. Wang, L. Zhang, S. Lee and H. Dai, Science, 2008, 319, 1229–1232 CrossRef CAS PubMed
.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 14095–14107 CrossRef CAS
.
- K. Habiba, V. I. Makarov, J. Avalos, M. J. F. Guinel, B. R. Weiner and G. Morell, Carbon, 2013, 64, 341–350 CrossRef CAS
.
- P. Lespade, R. Al-Jishi and M. Dresselhaus, Carbon, 1982, 20, 427–431 CrossRef CAS
.
- F. Tuinstra and J. L. Koenig, J. Chem. Phys., 1970, 53, 1126 CrossRef CAS
.
- M. S. Dresselhaus, A. Jorio, A. G. Souza Filho and R. Saito, Philos. Trans. R. Soc., A, 2010, 368, 5355–5377 CrossRef CAS PubMed
.
- C. Liu, P. Zhang, F. Tian, W. Li, F. Li and W. Liu, J. Mater. Chem., 2011, 21, 13163–13167 RSC
.
- R. Liu, D. Wu, S. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 48, 4598–4601 CrossRef CAS PubMed
.
- L. Zheng, Y. Chi, Y. Dong, J. Lin and B. Wang, J. Am. Chem. Soc., 2009, 131, 4564–4565 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19219f |
| This journal is © The Royal Society of Chemistry 2015 |