Triethylenetetramine modified multiwalled carbon nanotubes for the efficient preconcentration of Pb(ii), Cu(ii), Ni(ii) and Cd(ii) before FAAS detection
Abstract
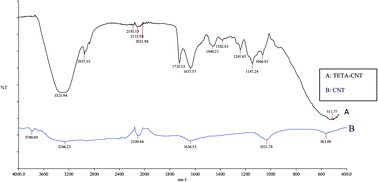
A novel, accurate and easy separation and preconcentration procedure has been established in the presented work. Triethylenetetramine modified multiwalled carbon nanotubes (TETA-MWCNTs) were prepared and used as an efficient adsorbent for the solid phase extraction of heavy metal ions. This modification of multiwalled carbon nanotubes results in the surfaces of MWCNTs being rich with amino groups, which have a strong interaction with heavy metal ions. The characterization and surface morphology of TETA-MWCNTs were evaluated using FT-IR and SEM. The parameters of the developed solid phase extraction procedure were investigated. The optimum results were obtained at pH 7, and with 8 mL of 3 M nitric acid as the eluent. The LOD values were 3.7 μg L−1, 0.5 μg L−1, 2.4 μg L−1 and 0.3 μg L−1 for Pb(II), Cu(II), Ni(II) and Cd(II), respectively. The relative standard deviations (RSDs) were found to be 1.4%, 4.7%, 2.9% and 4.2% for Pb(II), Cu(II), Ni(II) and Cd(II), respectively. This method was validated by using it for certified reference materials (TMDA-53.3 fortified water and TMDA-64.2 Lake Ontario water). The TETA-MWCNTs were applied for the preconcentration of Pb(II), Cu(II), Ni(II) and Cd(II) from water, cigarette and fertilizer samples.

 Please wait while we load your content...
Please wait while we load your content...