A solvent- and catalyst-free domino reaction for the efficient synthesis of 3-arylthiazolidine-2-thiones under microwave irradiation†
Abstract
A facile synthesis of 4-hydroxy-3-arylthiazolidine-2-thiones through novel domino reactions of aryl isothiocyanates and 1,4-dithiane-2,5-diol under solvent- and catalyst-free microwave irradiation is reported. This highly atom efficient reaction presumably proceeds via 2-mercaptoacetaldehyde generation/thia-Michael addition/regioselective hemiaminalization domino sequence. This reaction also proceeds in good yields with aryl isocyanates affording 3-phenylthiazol-2(3H)-ones.
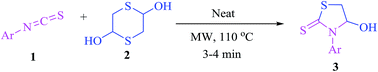

 Please wait while we load your content...
Please wait while we load your content...