Improved mechanical and tribological properties of bismaleimide composites by surface-functionalized reduced graphene oxide and MoS2 coated with cyclotriphosphazene polymer
Abstract
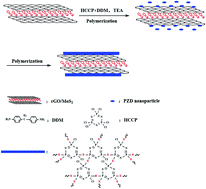
Surface-functionalized reduced graphene oxide and MoS2 hybrid nanosheets (rGO/MoS2) were obtained with a poly-(cyclotriphosphazene-co-4,4′-diaminodiphenylmethane) (PZD) polymer coating by a one-pot noncovalent method. The PZD/rGO/MoS2 hybrid nanoparticles were then chosen as fillers to improve the mechanical and tribological properties of bismaleimide (BMI) resin. The results showed that suitable addition of PZD/rGO/MoS2 could greatly enhance not only the mechanical and tribological properties but also the thermal stability. A low friction coefficient of 0.06 and volume wear rate of 1.80 × 10−6 mm3 (N m)−1 with 0.4 wt% PZD/rGO/MoS2 were obtained. This is mainly attributed to the unique layered structure of PZD/rGO/MoS2 hybrid nanoparticles, the enhanced toughness of the composites, good interfacial adhesion and compatibility between PZD/rGO/MoS2 and the BMI matrix, as well as the synergistic effect between nanosheets of rGO and MoS2.

 Please wait while we load your content...
Please wait while we load your content...