Experimental and theoretical investigations of Michelia alba leaves extract as a green highly-effective corrosion inhibitor for different steel materials in acidic solution†
Lingjie Lia,
Wenting Xua,
Jinglei Lei*a,
Junying Wanga,
Jianxin Hea,
Nianbing Lib and
Fusheng Panc
aSchool of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400044 P. R. China. E-mail: JLLei@cqu.edu.cn; Fax: +86 23 65112328; Tel: +86 13983064116
bSchool of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715 P. R. China
cSchool of Materials Science and Engineering, Chongqing University, Chongqing, 400044 P. R. China
First published on 26th October 2015
Abstract
The aqueous Michelia alba leaf extract (MALE) was first evaluated as an inhibitor to the corrosion of different steel materials (industrial pure iron, stainless steel and carbon steel) in hydrochloric acid. The adsorption and corrosion inhibition of MALE were investigated by potentiodynamic polarization, scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR) and quantum chemical calculations. The results showed that MALE acted as a highly-efficient mixed-type inhibitor for all steels and increasing temperatures benefited its corrosion inhibition. The adsorption of MALE on steel surfaces obeyed a Langmuir adsorption isotherm. Quantum chemical calculation results provided reasonable theoretical explanation for the inhibition property of MALE.
Introduction
Steels, e.g. industrial pure iron, stainless steel, carbon steel, etc., have wide applications in industry. All steels are prone to serious corrosion when they come into contact with acid solutions such as hydrochloric acid and sulfuric acid during acid pickling and cleaning processes.1–4 To protect steels from acid corrosion, many methods have been utilized, among which inhibition is one of the most facile and effective methods.5 The traditional inhibitors, such as synthetic organic compounds, are very effective in reducing corrosion of steels. However, most of them are expensive and highly toxic to both human beings and environment. Thus, the development and application of corrosion inhibitors are focused on the inexpensive and environmental-friendly substances. The plant extracts emerge out as green effective corrosion-inhibitors in recent years because of their low-cost, high availability, high biodegradability, and non-toxic nature.6,7 The leaves extracts of Occimum viridis,1 Hibiscus sabdariffa,1 thyme,8 Ochrosia oppositifolia,9 Acalypha torta10 and Piper guineense,11 barks extracts of Ochrosia oppositifolian9 and Schinopsis lorentzii,12 seed extracts of Phoenix dactylifera,13 root extract of Chlorophytum borivilianum,14 flowers extracts of Artemisia pallens15 and Tagetes erecta16 were reported as good inhibitors for mild-steel corrosion in aggressive acid solutions. The extracts of coffee ground,17 fruit peels,18 garlic peels,19 Oxandra asbeckii leaves,20 and Osmanthus fragran leaves2,21 were found to display strong inhibition for carbon-steel corrosion in acid solutions. The leaves extracts of ginkgo22 and bamboo3,4 were regarded as good corrosion inhibitors for cold-rolled steel in acid solutions and that of Salvia officinalis23 showed effective inhibition to the corrosion of stainless steel in hydrochloric acid solution. Obviously, these reported plant inhibitors can protect one certain steel from acid corrosion. However, to the best of our knowledge, no plant inhibitor has been reported to have general highly-efficient inhibition to acid corrosion of different steel materials. Therefore, developing new plant inhibitors with general highly-effective inhibition to corrosion of different steel materials in acid solutions is highly desirable in industrial applications.Michelia alba is a member of the magnoliaceae family of flowering plants, which are well known for the aroma constituents and bioactivities.24,25 Though the flowers of M. alba have demonstrated great economic importance, the leaves of M. alba have been rarely utilized and always treated as wastes. Constituents of M. alba leaves have been reported to include aporphines, oxoaporphines, sesquiterpenes, terpenes, benzenoids, steroids, and lignans as well as some aliphatic compounds such aspalmitic acid, stearic acid, and linoleic acid,26 which are rich in functional groups with oxygen and nitrogen atoms, epoxy groups and aromatic rings that meet the general structural consideration of corrosion inhibitors.6,7,27–29 Hence, it is inferred that M. alba leaves may find important application in corrosion inhibition.
In the present work, M. alba leaves extract (MALE) is first evaluated as a corrosion inhibitor for three different types steel materials (industrial pure iron, stainless steel and carbon steel) in hydrochloric acid. The adsorption and corrosion inhibition of MALE are investigated by the potentiodynamic polarization method, scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR) and quantum chemical calculations in detail. Such work on exploiting the utility of M. alba leaves in inhibition of steel corrosion is hopeful to expand the industrial applications of M. alba leaves and to develop new green inhibitors with more effective and general inhibition for acid-corrosion of different steel materials.
Results and discussion
Polarization curves
Fig. 1 and 2 illustrate the polarization curves of industrial-pure iron (a), 303 stainless steel (b) and Q235 carbon steel (c) in 1.0 M HCl solutions containing different concentrations of MALE at T1 (20 °C) and T2 (40 °C), respectively. The inhibition efficiency (η) can be calculated using the following equation:
 | (1) |
 | ||
| Fig. 1 Polarization curves of (a) industrial-pure iron, (b) 303 stainless steel, and (c) Q235 carbon steel in 1.0 M HCl with different concentrations of MALE at 20 °C. | ||
 | ||
| Fig. 2 Polarization curves of (a) industrial-pure iron, (b) 303 stainless steel, and (c) Q235 carbon steel in 1.0 M HCl with different concentrations of MALE at 40 °C. | ||
At both temperatures, the presence of MALE shifts both cathodic and anodic branches to the lower values of corrosion current densities and thus causes a remarkable decrease in the corrosion rate for the three different steel materials. The parameters, corrosion potential (Ecorr), Tafel slopes (bc and ba), corrosion current density (icorr) and inhibition efficiency (η) that derived from the polarization curves in Fig. 1 and 2 are given in Tables 1 and 2, respectively. As well known, different steel materials have dissimilar chemical compositions and microstructures, which result in their different corrosion behavior in acid solutions. Apparently, among the three types of steel materials, stainless steel is the least corrosive material while industrial-pure iron shows most serious corrosion at 20 °C and carbon steel shows most serious corrosion at 40 °C. However, regardless of steel types, icorr decreases considerably in the presence MALE and the inhibition efficiencies η are higher than 90% at 20 °C and 96% at 40 °C, which indicates that MALE is a highly-efficient inhibitor with general inhibition for different steel materials in acid solution.
| Steel materials | CMALE (g L−1) | Ecorr (mV vs. SCE) | bc (mV dec−1) | ba (mV dec−1) | icorr (μA cm−2) | η (%) |
|---|---|---|---|---|---|---|
| Industrial-pure iron | 0 | −512 | −103 | 68 | 1033 | — |
| 0.042 | −498 | −114 | 50 | 403 | 61.0 | |
| 0.084 | −510 | −114 | 64 | 318 | 69.2 | |
| 0.422 | −498 | −111 | 74 | 198 | 80.8 | |
| 0.843 | −494 | −125 | 65 | 150 | 85.5 | |
| 1.686 | −502 | −127 | 83 | 102 | 90.2 | |
| 303 stainless steel | 0 | −431 | −93 | 143 | 257 | — |
| 0.042 | −445 | −99 | 104 | 113 | 56.0 | |
| 0.084 | −441 | −94 | 76 | 70 | 73.0 | |
| 0.422 | −426 | −95 | 65 | 38 | 85.2 | |
| 0.843 | −422 | −95 | 58 | 31 | 87.9 | |
| 1.686 | −411 | −97 | 58 | 22 | 91.5 | |
| Q235 carbon steel | 0 | −448 | −109 | 57 | 583 | — |
| 0.042 | −463 | −118 | 47 | 186 | 68.1 | |
| 0.084 | −467 | −117 | 54 | 181 | 69.0 | |
| 0.422 | −465 | −113 | 68 | 106 | 81.8 | |
| 0.843 | −468 | −117 | 88 | 69 | 88.1 | |
| 1.686 | −471 | −113 | 86 | 54 | 90.8 |
| Steel materials | CMALE (g L−1) | Ecorr (mV vs. SCE) | bc (mV dec−1) | ba (mV dec−1) | icorr (μA cm−2) | η (%) |
|---|---|---|---|---|---|---|
| Industrial-pure iron | 0 | −493 | −141 | 78 | 6194 | — |
| 0.042 | −515 | −123 | 61 | 1435 | 76.8 | |
| 0.084 | −503 | −121 | 66 | 964 | 84.4 | |
| 0.422 | −510 | −119 | 80 | 584 | 90.6 | |
| 0.843 | −517 | −126 | 81 | 268 | 95.7 | |
| 1.686 | −509 | −128 | 76 | 205 | 96.7 | |
| 303 stainless steel | 0 | −438 | −125 | 189 | 5358 | — |
| 0.042 | −441 | −117 | 168 | 904 | 83.1 | |
| 0.084 | −440 | −111 | 137 | 538 | 90.0 | |
| 0.422 | −432 | −107 | 88 | 196 | 96.3 | |
| 0.843 | −424 | −97 | 72 | 129 | 97.6 | |
| 1.686 | −421 | −99 | 69 | 101 | 98.1 | |
| Q235 carbon steel | 0 | −456 | −156 | 91 | 8851 | — |
| 0.042 | −448 | −140 | 80 | 1991 | 77.5 | |
| 0.084 | −449 | −134 | 56 | 991 | 88.8 | |
| 0.422 | −468 | −124 | 78 | 402 | 95.5 | |
| 0.843 | −475 | −124 | 75 | 292 | 96.7 | |
| 1.686 | −484 | −130 | 82 | 138 | 98.4 |
The parallel cathodic polarization curves in Fig. 1 and 2 suggest that the hydrogen evolution is activation-controlled and the reduction mechanism is not affected by the presence of MALE.13,30,31 The anodic current decreases in the presence of MALE compared to its absence, which implies that MALE can also suppress the anodic dissolution of steels. Generally, an inhibitor can be classified as cathodic or anodic type if the shift of corrosion potential is more than 85 mV with respect to that in the inhibitor absence.13,30–32 From Fig. 1 and 2 together with Tables 1 and 2, the maximum shift of Ecorr is far less than 85 mV in the presence of MALE compared to its absence, which suggests that MALE acts as a mixed-type inhibitor. From the above results, it can be deduced that the inhibition of MALE might base upon the adsorption of its constituent molecules at the steel/solution interfaces while affect little on the mechanism of either the anodic metal dissolution or the cathodic hydrogen evolution reaction.
Adsorption behaviors
Generally, the adsorption process of organic molecules at a metal/solution interface can be regarded as the following replacement process of water molecules on the metallic surface (H2O(ads)):33| Org(sol) + xH2O(ads) ↔ Org(ads) + xH2O | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c), are commonly used to characterize the adsorption performance. The following Langmuir adsorption isotherm was found to be the one that best explains the experimental results for MALE:
c), are commonly used to characterize the adsorption performance. The following Langmuir adsorption isotherm was found to be the one that best explains the experimental results for MALE:
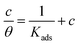 | (3) |
Fig. 3 shows the straight lines of c/θ vs. c at T1 (20 °C) and T2 (40 °C) for industrial-pure iron (a), 303 stainless steel (b) and Q235 carbon steel (c). Regardless of steel types, the linear correlation coefficients and the slopes are almost equal to 1 at both temperatures, which confirm that the adsorptions of MALE on the three steel surfaces all obey Langmuir adsorption isotherm. Usually, higher values of adsorptive equilibrium constant Kads imply better inhibition efficiency and more efficient adsorption.39 The values of Kads at 40 °C are all higher than those at 20 °C, which implies that the high temperature benefits the strong adsorption of MALE on steel surfaces. Thus, the inhibition efficiencies of MALE at 40 °C are higher than those at 20 °C can be well understood.
 | ||
| Fig. 3 Adsorption isotherms of MALE on surfaces of (a) industrial-pure iron, (b) 303 stainless steel, and (c) Q235 carbon steel at 20 °C and 40 °C. | ||
Moreover, the adsorption heat can be calculated according to the van't Hoff equation:35,36
 | (4) |
 | (5) |
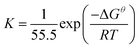 | (6) |
| Steel materials | Temp. (°C) | R2 | Kads (L g−1) | ΔHθ (kJ mol−1) | ΔGθ (kJ mol−1) |
|---|---|---|---|---|---|
| Industrial-pure iron | 20 | 0.999 | 24.9 | 30.4 | −17.6 |
| 40 | 1.000 | 55.3 | 30.4 | −20.9 | |
| 303 stainless steel | 20 | 0.999 | 29.9 | 51.8 | −18.1 |
| 40 | 0.999 | 116.3 | 51.8 | −22.8 | |
| Q235 carbon steel | 20 | 0.999 | 29.3 | 35.9 | −18.0 |
| 40 | 0.999 | 75.1 | 35.9 | −21.7 |
The negative values of ΔGθ (Table 3) indicate that the adsorptions of MALE on the steel surfaces are spontaneous. Generally, the values of ΔGθ around or less than −20 kJ mol−1 are associated with the electrostatic interaction between charged molecules and the charged metal surface (physisorption) while those around or higher than −40 kJ mol−1 mean charge sharing or transfer from the inhibitor molecules to the metal surface to form a coordinate type of metal bond (chemisorption).2,32,33 The ΔGθ values for the three different steel materials listed in Table 3 are all around −20 kJ mol−1, which means that the absorptions of MALE on the steel surfaces belong to physisorption and the adsorptive films have electrostatic characters.2,38,39
SEM results
The surface morphologies of industrial-pure iron (Fig. 4a and a′), 303 stainless steel (Fig. 4b and b′) and Q235 carbon steel (Fig. 4c and c′) samples after immersion in 1.0 M HCl solution in absence and presence of MALE (1.686 g L−1) for 1 h were examined by SEM and the corresponding images are shown in Fig. 4.In the absence of MALE (Fig. 4a–c), the surfaces of three steels display strongly-damaged morphologies with many pits and cracks, indicating the serious corrosion of steel materials in hydrochloric acid. While in the presence of MALE (Fig. 4a′–c′), the surfaces of three steels are much smoother as a consequence of formation of uniform and dense surface films due to adsorption of MALE, which significantly reduce the corrosion rate and provide strong protection for steel materials. These SEM results further confirm the results deduced from the potentiodynamic polarization tests that MALE is a highly-efficient inhibitor for the corrosion of steel materials.
FTIR results
Fig. 5 shows the FTIR spectra of MALE powder (Fig. 5a), and surface films formed on industrial-pure iron (Fig. 5b), 303 stainless steel (Fig. 5c), and Q235 carbon steel (Fig. 5d) after immersion in 1.0 M HCl containing 0.843 g L−1 MALE for 1 h. The corresponding functional groups assigned to the FTIR bands in Fig. 5 are identified in Table S2 in the ESI.† From Fig. 5 and Table S2 in the ESI,† it can be inferred that pure MALE powder contains oxygen and nitrogen atoms in functional groups (O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–N, C–O, C
O, C–N, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), epoxy group and aromatic ring, which is in agreement with the previous report on constituents of M. alba leaves.12,34,40–43 These functional groups are always found in constituents of plant corrosion inhibitors, which are considered to be crucial for the inhibition property.6,7,27–29 Compared with those of pure MALE powder, the FTIR results of surface films formed on different steel materials display some differences, e.g. some disappearing vibrations and shifting bands. These changes might be due to formation of complex of Fe2+-MALE adsorbing on steel surfaces, which is responsible for corrosion inhibition.
C), epoxy group and aromatic ring, which is in agreement with the previous report on constituents of M. alba leaves.12,34,40–43 These functional groups are always found in constituents of plant corrosion inhibitors, which are considered to be crucial for the inhibition property.6,7,27–29 Compared with those of pure MALE powder, the FTIR results of surface films formed on different steel materials display some differences, e.g. some disappearing vibrations and shifting bands. These changes might be due to formation of complex of Fe2+-MALE adsorbing on steel surfaces, which is responsible for corrosion inhibition.
Quantum chemical calculation results
Generally, a plant extract is considered as a complex mixture of various phytochemical components. According to the previous investigation on constituents of M. alba leaves24–26 and the above FTIR results, and further after some qualitative tests on inhibition property of each assumed component, gallic acid [GA], flavanoids [Fvo], ρ-hydroxybenzoic acid [ρ-HA] and liriodenine are identified as the major effective components in MALE. Hence, quantum chemical calculations are performed to model the adsorption structures of the above four chemical constituents (GA, Fvo, ρ-HA and liriodenine) in order to provide some insights into the nature of their interactions with steels and their contributions to inhibitions.Fig. 6 and 7 show the optimized structures, the highest occupied molecular orbitals (HOMO) and the lowest unoccupied molecular orbitals (LUMO) of GA, Fvo, ρ-HA and liriodenine. Clearly, HOMO of the four molecules is predominantly made up of N, O atoms and benzene rings.44 Table 4 lists some quantum chemical parameters, which are thought important due to their direct influence on electronic interactions between the inhibitor molecules and steel surfaces. EHOMO, energy of the highest occupied molecular orbital; ELUMO, energy of the lowest unoccupied molecular orbital; ΔE, energy gap between ELUMO and EHOMO; μ, dipole moment; 〈α〉, polarizability; η, hardness; σ, softness; Pi, electronic chemical potential; χ, electronegativity and ω, electrophilicity index. The η, σ, Pi, χ and ω parameters are calculated using the following equations:45–48
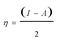 | (7) |
 | (8) |
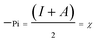 | (9) |
 | (10) |
| I = −EHOMO | (11) |
| A = −ELUMO | (12) |
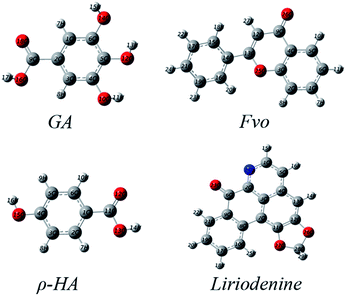 | ||
| Fig. 6 Optimized structures of molecules of the major effective components of MALE in the neutral form. | ||
| GA | Fvo | ρ-HA | Liriodenine | |
|---|---|---|---|---|
| EHOMO (eV) | −5.98 | −6.34 | −6.40 | −6.01 |
| ELUMO (eV) | −1.05 | −1.79 | −1.03 | −2.40 |
| ΔE (eV) | 4.93 | 4.55 | 5.37 | 3.61 |
| μ (D) | 2.41 | 4.19 | 1.85 | 6.94 |
| 〈α〉 | 88.01 | 164.24 | 79.17 | 199.56 |
| η (eV) | 2.46 | 2.28 | 2.68 | 1.80 |
| σ (eV−1) | 0.41 | 0.44 | 0.37 | 0.56 |
| Pi (eV) | −3.52 | −4.06 | −3.72 | −4.20 |
| χ (eV) | 3.52 | 4.06 | 3.72 | 4.20 |
| ω | 2.52 | 3.61 | 2.58 | 4.90 |
According to the frontier molecular orbital (FMO) theory,27,49 the electron transition is due to an interaction between HOMO and LUMO of the reacting species. EHOMO level is often associated with the electron donating ability of a molecule and the higher EHOMO value usually indicates a tendency of a molecule to donate electrons to the appropriate acceptor molecule with low energy and empty electron orbital. The value of ELUMO is related to the ability of a molecule to accept electrons and the lower ELUMO value often means that the molecule likely accepts electrons. Thus, the ΔE value is regarded as a measure for the stability of a formed complex on metal surface and the lower ΔE value reflects the higher stability of a formed complex.27,50 The dipole moment μ is a measure of the polarity of a covalent bond, which is related to the distribution of electrons in a molecule.50,51 Generally, the larger μ value favors the adsorption of inhibitor.51 The polarizability 〈α〉 is an indicator of the linear response of the electron density in the presence of an infinitesimal electric field, which depends on the second derivative of energy with respect to the electric field.44,52 The higher 〈α〉 value facilitates the strong adsorption process and high inhibition efficiency.52 The hardness η and softness σ can also reflect the ability of a molecule to accept and offer electrons. For the simplest transfer of electrons, adsorption could occur at the part of the molecule where σ has the highest value and η has the lowest value.53 The electrophilicity index ω is a measure of the electrophilic power of a molecule, the higher value of which means the higher capacity of a molecule to accept electrons.47,53
From Table 4, the EHOMO values of GA, Fvo, ρ-HA and liriodenine show very small differences (less than 0.42 eV), which indicates that these molecules have similar abilities to donate electrons to metallic surface.54 Liriodenine has the lowest ELUMO (−2.40 eV) and ΔE (3.62 eV), which implies the high capacity to accept electrons from the d-orbital of Fe and the high stability of the [Fe–liriodenine] complexes.55 Moreover, liriodenine has the highest values of dipole moment μ and polarizability 〈α〉 among these molecules, which implies the strong adsorption of liriodenine molecules at steel surfaces.
As for the η and σ parameters, the lowest η value and highest σ value of liriodenine indicate the highly effective inhibition of liriodenine to steel corrosion, which agrees well with other theoretical reports.27,56,57 Liriodenine has the highest ω value, which further confirms its high capacity to accept electrons and adsorb on steel surfaces to inhibit corrosion.
The above calculation results and discussion are propitious to the four major effective components in the neutral form. Further considering that the protonation of those four components might occur in acid medium, the calculations for the protonated form are performed. Fig. S2 and S3 in the ESI† show the optimized structures, the highest occupied molecular orbitals (HOMO) and the lowest unoccupied molecular orbitals (LUMO) of GA, Fvo, ρ-HA and liriodenine in the protonated form. Table S3 in the ESI† lists the related quantum chemical parameters.
It is evident that the protonated form of the four major effective components shows similar tendency to their neutral form. Liriodenine exhibits the highest EHOMO, and the lowest ELUMO and ΔE, suggesting the protonated form is the most likely form for its interaction with steels.58–60 Moreover, the protonated liriodenine has the lowest value of hardness η, and the highest value of softness σ and polarizability 〈α〉, indicating that liriodenine has the best inhibitive performance on the steel surface in the protonated form.61,62 Besides, the protonated liriodenine has the highest ω value, confirming that liriodenine in the protonated form is the most reactive inhibitor that can easily adsorb on the steel surface to provide stronger protection.15,63 In addition, the protonated GA has the highest values of dipole moment μ among all those four molecules, implying that GA has a good inhibitive performance to steel corrosion in the protonated form. All those results and deductions are in consistent with those calculated in case of the neutral form. However, the protonated ones make greater contribution than their neutral counterparts.
Considering all the calculated parameters and the molecule structures in the neutral and protonated forms, liriodenine is presumed to play the most important role in the inhibition performance of MALE, and Fvo and GA also make big contributions while ρ-HA has the least inhibition effect. However, since these molecules differ considerably in their chemical structures and any of them might exert a dominant effect under specific conditions and concentrations.
Experimental
Materials and solutions
Tests were performed on three distinct steel materials with obvious different compositions and applications: industrial-pure iron, 303 stainless steel and Q235 carbon steel (Rong Chuang Metal Co., Ltd, Dongguan, PR China). The compositions of the above steel materials are listed in Table S1 of the ESI.† The aggressive solution (1.0 M HCl) was prepared by dilution of 37% hydrochloric acid (analytical grade; Sinopharm Chemical Reagent Co., Ltd, Shanghai, PR China) with distilled water.Fresh M. alba leaves (Fig. S1 in the ESI†) were picked in the campus of Chongqing University in PR China. The aqueous MALE was prepared in the following way: 60 g fresh M. alba leaves were finely shredded and heated in boiled distilled water for 1 h. The mixture was filtered and the clear liquid was concentrated, dried in an oven to gain the dark-brown solid and then ground to obtain the extract powder, whose weight was 7.580 g. The extract powder was then added into the aggressive solution (1.0 M HCl) to prepare the test solutions with the desired MALE concentration. The concentration range of MALE used was 0.042–1.686 g L−1.
Electrochemical measurements
The inhibition efficiency of MALE was evaluated by using potentiodynamic polarization measurements, which were carried out on a CHI760B electrochemical workstation (Shanghai Chenhua Instruments Inc., PR China). The electrochemical cell consisted of a conventional three-electrode configuration with a platinum sheet as the counter electrode, a saturated calomel electrode (SCE) coupled with a Luggin–Haber capillary as the reference electrode, the different steel electrodes as the working electrodes, and 1.0 M HCl solutions with different concentrations of MALE as the electrolytes. The tip of the Luggin capillary was very close to the working electrode surface in order to minimize ohmic contributions. The steel working electrodes were cut from the above three steel rods with cross-section area of 1 cm2 and embedded in epoxy resin holders. Before each experiment, the surface of the working electrode was abraded with 200, 600, 800 and 1200# grit emery papers, then degreased ultrasonically in absolute ethanol for 3 min, rinsed with distilled water and dried in air. Before each measurement, the working electrode was immersed in the quiescent test solution (open to air) until the cell open circuit potential (OCP) became stable. The polarization curves were recorded from −0.30 V (vs. OCP) to +0.30 V (vs. OCP) at a sweep rate of 0.5 mV s−1. Each test was repeated more than three times to verify reproducibility of the results.SEM and FTIR characterizations
The surface morphology of different steel samples after immersion in 1.0 M HCl solution in absence and presence of MALE (with the concentration of 1.686 g L−1) at 20 °C for 1 h was examined by a scanning electron microscope (SEM, TESCAN VEGAII LMU, Czech).FTIR spectra of four specimens were recorded by using a Fourier transform infrared spectrometer (FTIR, Nicolet 60–SXB, USA), which extended from 400 to 4000 cm−1, using the KBr disk technique. One specimen for FTIR characterization was the MALE powder, which was mixed with KBr and made into the disk. Other three specimens were the thin adsorption layers formed on three different steel surfaces after immersion in 1.0 M HCl solution containing 0.843 g L−1 MALE at 20 °C for 1 h, which was cleaned and dried first and then rubbed with a small amount of KBr powder and made into the disk.
Quantum chemical calculations
The quantum theoretical calculations were carried out by using Gaussian 09 program. The full geometry optimization together with the vibrational analysis of the optimized structures was carried out at the DFT (density functional theory) B3LYP level using 6-31G* basis set to ensure that the optimized structures were corresponded to the minimum of the potential energy surface (no imaginary frequency). The quantum chemical parameters were then obtained from the optimized structure using 6-31G* basis set in gas phase. It has been proposed that carrying out the theoretical calculations in gas phase is a useful method because the results obtained in gas phase show no remarkable differences from those obtained in liquid phase while it can significantly reduce the time of calculation.64–69Conclusions
(1) The electrochemical polarization results indicate that MALE acts as a highly-efficient mixed-type inhibitor for corrosion of different steel materials in acid solution and increasing temperature benefits its corrosion inhibition.(2) The adsorptions of MALE on three steel surfaces all obey the Langmuir adsorption isotherm.
(3) The uniform and dense adsorptive films over three steel surfaces efficiently inhibit the corrosion of steel materials.
(4) The quantum-chemical calculations are performed by taking into consideration both the protonated and the nonprotonated species, which indicates that the component liriodenine plays the most important role in the inhibition performance of MALE.
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (21273293, 21373281, 21573028), the Program for New Century Excellent Talents in University (NCET-12-0587, NCET-13-0633), the Project for Distinguished Young Scholars in Chongqing (cstc2014jcyjjq100004), the Program of China Scholarships Council (No. 201406055006), the Fundamental Research Funds for the Central Universities (CDJZR13225501, CDJZR14228801) and the sharing fund of Chongqing University's Large-scale Equipment. The authors also thank Prof. Mingli Yang in Institute of Atomic and Molecular Physics of Sichuan University of P. R. China for supporting Gaussian 09 program as well as for the instructive discussion on quantum chemical calculations.Notes and references
- E. E. Oguzie, Corros. Sci., 2008, 50, 2993 CrossRef CAS.
- L. J. Li, X. P. Zhang, J. L. Lei, J. X. He, S. T. Zhang and F. S. Pan, Corros. Sci., 2012, 63, 82 CrossRef CAS.
- X. H. Li, S. D. Deng, H. Fu and X. G. Xie, Corros. Sci., 2014, 78, 29 CrossRef CAS.
- X. H. Li, S. D. Deng and H. Fu, Corros. Sci., 2012, 62, 163 CrossRef CAS.
- V. S. Sastri, Corrosion Inhibitors–Principles and Applications, Wiley, New York, 1st edn, 1998 Search PubMed.
- P. B. Raja and M. G. Sethuraman, Mater. Lett., 2008, 62, 113 CrossRef CAS.
- M. Finšgar and J. Jackson, Corros. Sci., 2014, 86, 17 CrossRef.
- T. Ibrahim, H. Alayan and Y. A. Mowaqet, Prog. Org. Coat., 2012, 75, 456 CrossRef CAS.
- P. B. Raja, M. Fadaeinasab, A. K. Qureshi, A. A. Rahim, H. Osman, M. Litaudon and K. Awang, Ind. Eng. Chem. Res., 2013, 52, 10582 CrossRef CAS.
- P. M. Krishnegowda, V. T. Venkatesha, P. K. M. Krishnegowda and S. B. Shivayogiraju, Ind. Eng. Chem. Res., 2013, 52, 722 CrossRef CAS.
- E. E. Oguzie, C. B. Adindu, C. K. Enenebeaku, C. E. Ogukwe, M. A. Chidiebere and K. L. Oguzie, J. Phys. Chem. C, 2012, 116, 13603 CAS.
- H. Gerengi and H. I. Sahin, Ind. Eng. Chem. Res., 2012, 51, 780 CrossRef.
- S. A. Umoren, Z. M. Gasem and I. B. Obot, Ind. Eng. Chem. Res., 2013, 52, 14855 CrossRef CAS.
- G. Ji, P. Dwivedi, S. Sundaram and R. Prakash, Ind. Eng. Chem. Res., 2013, 52, 10673 CrossRef CAS.
- S. Garai, S. Garai, P. Jaisankar, J. K. Singh and A. Elango, Corros. Sci., 2012, 60, 193 CrossRef CAS.
- P. Mourya, S. Banerjee and M. M. Singh, Corros. Sci., 2014, 85, 352 CrossRef CAS.
- V. V. Torres, R. S. Amado, C. F. de Sá, T. L. Femandez, C. A. Da Silva Riehl, A. G. Torres and E. D’Elia, Corros. Sci., 2011, 53, 2385 CrossRef CAS.
- J. C. da Rocha, J. A. da Cunha Ponciano Gomes and E. D'Elia, Corros. Sci., 2010, 52, 2341 CrossRef.
- S. S. de Assunção Araújo Pereira, M. M. Pêgas, T. L. Fernández, M. Magalhães, T. G. Schöntag, D. C. Lago, L. F. de Senna and E. D’Elia, Corros. Sci., 2012, 65, 360 CrossRef.
- M. Lebrini, F. Robert, A. Lecante and C. Roos, Corros. Sci., 2011, 53, 687 CrossRef CAS.
- L. J. Li, X. P. Zhang, J. L. Lei, J. X. He, S. T. Zhang and F. S. Pan, Asian J. Chem., 2012, 24, 1649 CAS.
- S. D. Deng and X. H. Li, Corros. Sci., 2012, 55, 407 CrossRef CAS.
- N. Soltani, N. Tavakkoli, M. Khayatkashani, M. R. Jalali and A. Mosavizade, Corros. Sci., 2012, 62, 122 CrossRef CAS.
- O. Rangasamy, G. Raoelison, F. E. Rakotoniriana, K. Cheuk, S. Urverg-Ratsimamanga, J. Quetin-Leclercq, A. Gurib-Fakim and A. H. Subratty, J. Ethnopharmacol., 2007, 109, 331 CrossRef.
- M. S. Choi, M. S. Yoo, D. J. Son, H. Y. Jung, S. H. Lee, J. K. Jung, B. C. Lee, Y. P. Yun, H. B. Pyo and J. T. Hong, J. Dermatol. Sci., 2007, 46, 127 CrossRef CAS.
- C. Y. Chen, L. Y. Huang, L. J. Chen, W. L. Lo, S. Y. Kuo, Y. D. Wang, S. H. Kuo and T. J. Hsieh, Chem. Nat. Compd., 2008, 44, 137 CrossRef CAS.
- G. Gece, Corros. Sci., 2008, 50, 2981 CrossRef CAS.
- G. Gece, Corros. Sci., 2011, 53, 3873 CrossRef CAS.
- B. Sanyal, Prog. Org. Coat., 1981, 9, 165 CrossRef CAS.
- R. Baskar, D. Kesavan, M. Gopiraman and K. Subramanian, Prog. Org. Coat., 2014, 77, 836 CrossRef CAS.
- M. R. Heydarpour, A. Zarrabi, M. M. Attar and B. Ramezanzadeh, Prog. Org. Coat., 2014, 77, 160 CrossRef CAS.
- D. Thirumoolan, V. A. Katkar, G. Gunasekaran, T. Kanai and K. A. Basha, Prog. Org. Coat., 2014, 77, 1253 CrossRef CAS.
- M. Behpour, S. M. Ghoreishi, A. Gandomi-Niasar, N. Soltani and M. Salavati-Niasari, J. Mater. Sci., 2009, 44, 2444 CrossRef CAS.
- M. V. Fiori-Bimbi, P. E. Alvarez, H. Vaca and C. A. Gervasi, Corros. Sci., 2015, 92, 192 CrossRef CAS.
- X. H. Li, S. D. Deng, H. Fu and G. N. Mu, Corros. Sci., 2010, 52, 1167 CrossRef CAS.
- T. P. Zhao and G. N. Mu, Corros. Sci., 1999, 41, 1937 CrossRef CAS.
- R. Baskar, D. Kesavan, M. Gopiraman and K. Subramanian, RSC Adv., 2013, 3, 17039 RSC.
- F. M. Donahue and K. Nobe, J. Electrochem. Soc., 1965, 112, 886 CrossRef CAS.
- M. Shabani-Nooshabadi, M. Behpour, F. S. Razavi, M. Hamadanian and V. Nejadshafiee, RSC Adv., 2015, 5, 23357 RSC.
- X. H. Li, S. D. Deng and H. Fu, J. Appl. Electrochem., 2010, 40, 1641 CrossRef CAS.
- X. H. Li, S. D. Deng, H. Fu and G. N. Mu, J. Appl. Electrochem., 2009, 39, 1125 CrossRef CAS.
- S. Manov, A. M. Lamazouere and L. Aries, Corros. Sci., 2000, 42, 1235 CrossRef CAS.
- A. P. Srikanth, T. G. Sunitha, V. Raman, S. Nanjundan and N. Rajendran, Mater. Chem. Phys., 2007, 103, 241 CrossRef CAS.
- F. Kandemirli and S. Sagdinc, Corros. Sci., 2007, 49, 2118 CrossRef CAS.
- R. G. Pearson, Inorg. Chem., 1988, 27, 734 CrossRef CAS.
- M. J. S. Dewar and W. Thiel, J. Am. Chem. Soc., 1977, 99, 4907 CrossRef CAS.
- R. G. Parr, L. v. Szentpaly and S. Liu, J. Am. Chem. Soc., 1999, 121, 1922 CrossRef CAS.
- V. S. Sastri and J. R. Perumareddi, Corros. Sci., 1997, 53, 617 CrossRef CAS.
- I. B. Obot and N. O. Obi-Egbedi, Colloids Surf., A, 2008, 330, 207 CrossRef CAS.
- N. O. Eddy and B. I. Ita, J. Mol. Model., 2011, 17, 359 CrossRef CAS PubMed.
- S. W. Xia, M. Qiu, L. M. Yu, F. G. Liu and H. Z. Zhao, Corros. Sci., 2008, 50, 2021 CrossRef CAS.
- M. A. Amin, M. A. Ahmed, H. A. Arida, F. Kandemirli, M. Saracoglu, T. Arslan and M. A. Basaran, Corros. Sci., 2011, 53, 1895 CrossRef CAS.
- H. Jafari, I. Danaee, H. Eskandari and M. RashvandAvei, Ind. Eng. Chem. Res., 2013, 52, 6617 CrossRef CAS.
- L. M. Rodríguez-Valdez, A. Martínez-Villafañe and D. Glossman-Mitnik, J. Mol. Struct., 2005, 716, 61 CrossRef.
- E. E. Oguzie, C. K. Enenebeaku, C. O. Akalezi, S. C. Okoro, A. A. Ayuk and E. N. Ejike, J. Colloid Interface Sci., 2010, 349, 283 CrossRef CAS PubMed.
- Y. L. Li, Q. P. Qin, Y. C. Liu, Z. F. Chen and H. Liang, J. Inorg. Biochem., 2014, 137, 12 CrossRef CAS PubMed.
- C. Y. Chen, S. Y. Chen and C. H. Chen, Process Biochem., 2012, 47, 1460 CrossRef CAS.
- D. Daoud, T. Douadi, S. Issaadi and S. Chafaa, Corros. Sci., 2014, 79, 50 CrossRef CAS.
- M. Faustin, A. Maciuk, P. Salvin, C. Roos and M. Lebrini, Corros. Sci., 2015, 92, 287 CrossRef CAS.
- G. Ji, S. Anjum, S. Sundaram and R. Prakash, Corros. Sci., 2015, 90, 107 CrossRef CAS.
- M. S. Masoud, M. K. Awad, M. A. Shaker and M. M. T. El-Tahawy, Corros. Sci., 2010, 52, 2387 CrossRef CAS.
- R. Goel, W. A. Siddiqi, B. Ahmed, M. S. Khan and V. M. Chaubey, Desalination, 2010, 263, 45 CrossRef CAS.
- D. Özkır, K. Kayakırılmaz, E. Bayol, A. A. Gürten and F. Kandemirli, Corros. Sci., 2012, 56, 143 CrossRef.
- I. B. Obot and N. O. Obi-Egbedi, Corros. Sci., 2010, 52, 657 CrossRef CAS.
- L. M. Rodríguez-Valdez, W. Villamisar, M. Casales, J. G. González-Rodriguez, A. Martínez-Villafañe, L. Martinez and D. Glossman-Mitnik, Corros. Sci., 2006, 48, 4053 CrossRef.
- M. S. Masoud, M. K. Awad, M. A. Shaker and M. M. T. El-Tahawy, Corros. Sci., 2010, 52, 2387 CrossRef CAS.
- J. J. Fu, H. S. Zang, Y. Wang, S. N. Li, T. Chen and X. D. Liu, Ind. Eng. Chem. Res., 2012, 51, 6377 CrossRef CAS.
- K. F. Khaled and M. A. Amin, J. Appl. Electrochem., 2009, 39, 2553 CrossRef CAS.
- K. F. Khaled, J. Appl. Electrochem., 2011, 41, 423 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19088f |
| This journal is © The Royal Society of Chemistry 2015 |



