A comparative investigation of carbon black (Super-P) and vapor-grown carbon fibers (VGCFs) as conductive additives for lithium-ion battery cathodes
Abstract
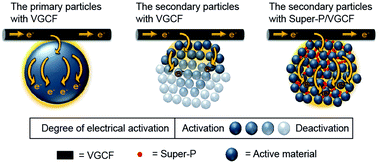
To investigate the synergistic effect of different types of conductive additives on the cathode performance of lithium-ion batteries, various types of cathode materials containing different ratios of vapor-grown carbon fibers (VGCFs) and carbon black (Super-P) are investigated. The pillar-like morphology of the VGCFs enabled them to efficiently connect to the active materials and hence, the highest electrical conductivity of LiCoO2 and LiFePO4 (both of which are composed of primary particles) was achieved with the VGCFs. On the other hand, for LiNi0.6Co0.2Mn0.2O2, composed of micro-sized secondary particles embedded with nano-sized primary particles, improved electrical conductivity was achieved with a mixture of VGCF and Super-P via synergistic action.

 Please wait while we load your content...
Please wait while we load your content...