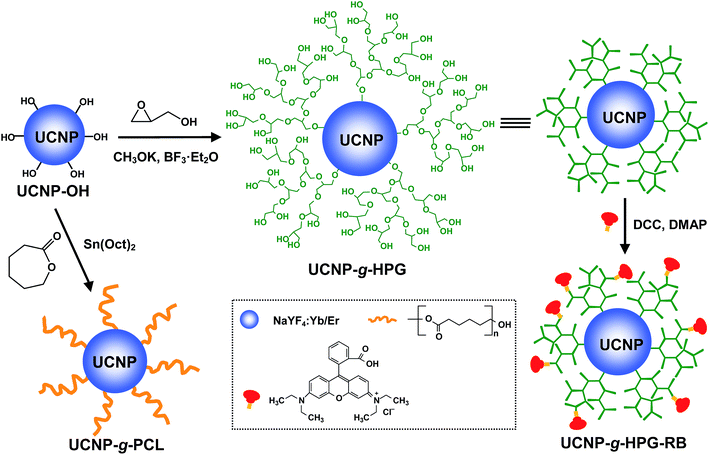
Efficient tailoring of the surface of upconversion nanoparticles via surface-initiated cationic ring-opening polymerization†
Benzhao He and
Li Zhou*
Guangxi Ministry-Province Jointly-Constructed Cultivation Base for State Key Laboratory of Processing for Nonferrous Metal and Featured Materials, Key Laboratory of New Processing Technology for Nonferrous Metal and Materials (Ministry of Education), College of Material Science and Engineering, Guilin University of Technology, Guilin 541004, P. R. China. E-mail: zhouli@glut.edu.cn
First published on 9th November 2015
Abstract
Decoration of lanthanide-doped upconversion nanoparticles (UCNPs) with polymers to endow the UCNPs with the desired properties for various applications has attracted considerable attention. Herein, we present for the first time that the surface-initiated cationic ring-opening polymerization (CROP) technique can be employed as an effective tool to decorate UCNP with diverse polymers, as exemplified by the successful grafting of hydrophobic poly(ε-caprolactone) and hydrophilic hyperbranched polyglycerol from the surface of NaYF4:Yb/Er UCNP. Furthermore, the content of the grafted polymer associated with the thickness of the polymer shell can be controlled by adjusting the feed ratio. Benefiting from the introduction of the polymer shell, the resulting UCNP/polymer nanohybrids simultaneously show strong upconversion luminescence, tunable solution dispersibility, enhanced solution processibility, high biocompatibility, and favorable surface functionality, which make them promising for various applications such as fabrication of light-emitting polymer composites, cellular imaging, and drug delivery. Considering the high robustness and flexibility of the CROP technique, this investigation opens up new avenues for decorating UCNPs with diverse polymers to meet various application requirements.
1 Introduction
Rare-earth upconversion nanoparticles (UCNP) that can convert low energy longer wavelength excitation into high energy shorter wavelength emission, is emerging as a promising luminescent nanomaterial for applications ranging from bioimaging to light-emitting diodes.1–4 The UCNPs have displayed advantages over small-molecule chromophores, fluorescent proteins and semiconductor quantum dots, which include a narrow emission peak, high photostability, deep penetration depth, very low auto-fluorescence background, and low toxicity.5–8 To facilitate the applications of UCNPs, tailoring their surface with polymers is often needed.9–12 The polymer shell can not only promote the solution dispersibility and stability of UCNP but also endow UCNP with new properties including favorable surface functionality, high biocompatibility, and good processability.The UCNP/polymer nanohybrids could be straightforwardly fabricated by employing hydrophilic polymers as ligands during the formation of UCNPs.13–15 In addition, a commonly used strategy is to tailor the pre-synthesized UCNPs with polymers via various techniques such as ligand attraction, ligand exchange, layer-by-layer (LBL) self-assembly, and host–guest interaction.16–25 Although significant progress has been made towards UCNP/polymer nanohybrids in recent years, several challenges remain to be overcome. Firstly, most of the previous polymer shells were constructed by non-covalent interactions, and thus the resulted UCNP/polymer nanohybrids would display poor stability during applications. Secondly, the thickness of polymer shell associated with the surface properties of the nanohybrids is hard to manipulate by most of the previous reports. Although the LBL self-assembly technique can afford nanohybrids with tunable shell thickness, only specific polymers are suitable for this approach.25 On the other hand, in situ growth of polymer shell on the surface of inorganic nanomaterial by surface-initiated living/controlled polymerization technique has been widely used to produce nanohybrids with controllable thickness of polymer shell.26–31 However, this method has rarely been employed to decorate UCNPs with polymers.32
In this contribution, we present a simple and viable approach to covalently functionalize NaYF4:Yb/Er UCNPs with polymer shells by surface-initiated cationic ring-opening polymerization (CROP) technique (Scheme 1). To our knowledge, this is the first time to employ surface-initiated CROP technique to decorate UCNPs with polymer shells. Two representative biocompatible polymers including hydrophobic poly(ε-caprolactone) (PCL) and hydrophilic hyperbranched polyglycerol (HPG) were directly grown on the surface of UCNPs to afford UCNP-g-PCL and UCNP-g-HPG nanohybrids, respectively. Benefiting from the presence of polymer shells, the obtained nanohybrids exhibited markedly enhanced solution dispersibility, favorable processibility, and low cytotoxicity. Meanwhile, the polymer shell can provide numerous reactive functional groups to conjugate functional molecules for further applications, as exemplified by the conjugation and release of rhodamine B (RB) model drug molecule in this paper. From the perspective of material science, controllable and covalent grafting of polymers from the surface of UCNPs also offers a chance to study the influence of polymers on the applied properties of UCNPs.
2 Experimental section
2.1 Materials
Rare earth chlorides (LnCl3·6H2O, Ln: Y, Yb, Er), ricinoleic acid, 1-octadecene, ammonium fluoride, ε-caprolactone (ε-CL), glycidol, boron trifluoride diethyl etherate (BF3·Et2O), tin(II)-2-ethylhexanoate (Sn(Oct)2), 4-(dimethylamino)pyridine (DMAP), N,N′-dicyclohexylcarbodiimide (DCC), rhodamine B (RB) and lipase were purchased from Aladdin Chemistry Co. Ltd. (Shanghai, China) and used as received. Glycidol and ε-caprolactone were dried over CaH2 at room temperature for 48 h and distilled under reduced pressure. All other chemicals were of analytical grade and were used as received without further purification. Milli-Q water (18.2 MΩ) was used for all experiments. The hydroxyl-functionalized NaYF4:Yb/Er upconversion nanoparticle (UCNP-OH) was synthesized according to our previous report.332.2 Characterization
Fourier transform infrared (FTIR) spectra were recorded on a Thermo Nexus 470 FTIR spectrometer (KBr disk). Thermogravimetric analysis (TGA) was conducted on a Netzsch STA 449C analyzer with a heating rate of 20 °C min−1 under a nitrogen flow. Transmission electron microscopy (TEM) images were recorded on a JEM-2100 F high resolution TEM at 160 kV. Samples were prepared by placing a drop of a dilute aqueous dispersion on the surface of a copper grid. Dynamic light scattering (DLS) measurements were carried out in water dispersion using a Malvern Zetasizer Nano ZS90 apparatus. Upconversion emission spectra were measured on a VARIAN fluorescence spectrophotometer equipped with a 0–800 mW adjustable continuous-wave laser (980 nm, Beijing Hi-Tech Optoelecronic Co., China). Absorption spectra were recorded on a UV-3600 UV-vis-near-IR spectrophotometer (Shimadzu).2.3 Synthesis of UCNP-g-PCL via surface-initiated CROP technique
Typically, UCNP-OH initiator (80 mg), ε-CL (640 mg) and Sn(Oct)2 (0.5 μL) were placed in a dry flask, which was purged with argon for 30 min to remove the oxygen and then sealed with a rubber plug. The flask was immersed in an oil bath at 120 °C and stirred for 24 h. After cooling to room temperature, the mixture was dissolved in dichloromethane (DCM) and subsequently centrifuged and washed several times with DCM to completely remove ungrafted PCL. After repeated washing and centrifugation steps, the resulting solid product (designated as UCNP-g-PCL) was dried overnight under vacuum at 50 °C.2.4 Synthesis of UCNP-g-HPG via surface-initiated CROP technique
In a typical procedure, UCNP-OH (50 mg), BF3·Et2O (30 μL) and anhydrous DCM (15 mL) were added into a flask. The flask was placed in an ultrasonic bath for 15 min to ensure complete suspension of the UCNP-OH initiator. Then the flask was purged with argon for 30 min to remove the oxygen. The system was immersed in a low temperature reactor with the reaction temperature kept constant at −20 °C throughout the polymerization process. Glycidol (2 mL) was added dropwise over a period of 8 h. After completion of monomer addition, the mixture was stirred for an additional 4 h. After reaction, the mixture was centrifuged and washed several times with methanol. After repeated washing and centrifugation steps, the resulting solid product (designated as UCNP-g-HPG) was dried overnight under vacuum at 50 °C.2.5 Synthesis of UCNP-g-HPG-RB
The RB dye was chosen as a model molecule to conjugate UCNP-g-HPG. Typically, 20 mg of UCNP-g-HPG, 5 mg of RB, and 10 mL of DMF were placed in a dry flask and treated in an ultrasonic bath for 5 min. Subsequently, 18.6 mg of DCC and 5 mg of DMAP were added to the flask, and the mixture was stirred at 50 °C for 24 h. The product was separated by centrifugation and washed with ethanol repeatedly until the washed ethanol became colorless. After being dried overnight under vacuum, the product (designated as UCNP-g-HPG-RB) was obtained.2.6 Release of RB from UCNP-g-HPG-RB
The release of RB was monitored by UV-vis spectrophotometer at λmax = 562 nm as a function of time. Calibration curves were plotted between absorbance and concentration of the RB solutions. The release behaviors of RB from the UCNP-g-HPG-RB in the presence of lipase were studied at 37 °C in a medium (pH 7.4, containing 1 mM MgCl2 and 50 mM KCl). UCNP-g-HPG-RB (2 mg mL−1) and lipase (5 mg mL−1) was dispersed in the medium. As a control, the release profiles of RB without lipase were also studied. The mixture was placed at 37 °C with constant stirring. At predetermined intervals, the release medium was collected by centrifugation for analysis by UV-vis spectrophotometer. Each experiment was carried out in triplicate. Release% = (the amount of RB released from UCNP-g-HPG-RB)/(the total amount of RB loaded by UCNP-g-HPG-RB) × 100%.2.7 Cytotoxicity evaluation
The in vitro cytotoxicity of UCNP-g-HPG was examined by using methyl-thiazolyldiphenyl-tetrazolium (MTT) assay. NIH-3T3 mouse embryo fibroblast cells were seeded in 96-well plates at 1 × 104 cells per well. After 24 h incubation, the medium was replaced by the sample solutions with concentration of 0, 50, 100, 200, and 500 μg mL−1, respectively. The cells were then incubated for 24 h or 48 h. After specific time intervals, the wells were washed twice with PBS buffer. Subsequently, 10 μL of freshly prepared MTT (5 mg mL−1) solution was added to each well. After 3 h incubation, the MTT medium solution was removed. Dimethyl sulphoxide (0.1 mL) was then added into each well, and the plate was shaken for 15 min at room temperature. Cell viability was calculated by the ratio of absorbance (570 nm) of the cells incubated with sample solution to that of the cells incubated with culture medium only.2.8 Cell imaging
MCF-7 cells were cultured in a RPMI 1640 medium containing 10% fetal bovine serum and 1% penicillin–streptomycin at 37 °C in a humidified environment of 5% CO2. After 80% confluence, the medium was removed and the adherent cells were washed twice with phosphate buffer. The UCNP-g-HPG solution (50 μg mL−1, 0.4 mL) was added to the chamber. After incubation for 2 h, cells were washed three times with phosphate buffer and then fixed by 75% ethanol for 20 min, which was further washed twice with phosphate buffer. The cells were imaged by a confocal laser scanning microscope (CLSM, Nikon-A1) equipped with a 980 nm NIR laser and a Nikon digital camera.3 Results and discussion
3.1 Synthesis and characterization of UCNP-g-PCL
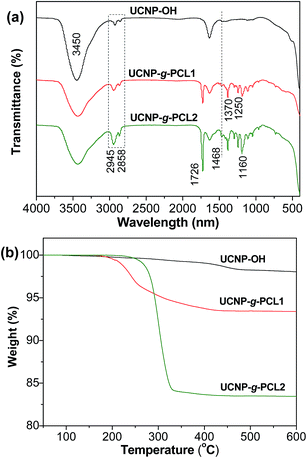
PCL as one of the most crucial biodegradable and biocompatible polymers can grant access to the potential applications for UCNP-g-PCL in the fields of bionanotechnology.34 In order to grow PCL shell on the surface of UCNP by surface-initiated CROP technique, a prerequisite is to obtain UCNP with hydroxyl initiators (UCNP-OH).35–37 Very recently, our group developed an easy method to synthesize UCNP-OH by using ricinoleic acid as ligand through solvothermal reaction.33 TEM image indicates that the size of UCNP-OH is in the range of 22–30 nm (Fig. 1a). Three strong bands at 2945, 2858 and 1468 cm−1 corresponding to C–H vibration, and two obvious bands at around 1150 and 3450 cm−1 respectively associated with C–O–C and –OH groups are seen in the FTIR spectrum of UCNP-OH (Fig. 2a and S1†), demonstrated the successful introduction of hydroxyl initiators onto the surface of UCNP. The content of hydroxyl initiator was measured by thermo gravimetric analysis (TGA). The UCNP-OH sample presents a weight-loss of 1.94 wt% between 150–600 °C (Fig. 2b), further confirmed the presence of ricinoleic acid ligand on the surface of UCNP and the corresponding density of –OH groups is ca. 0.065 mmol g−1. Such a relatively high content of –OH initiator provides a good platform for performing surface-initiated CROP.Tin(II)-2-ethylhexanoate as a catalyst was employed to initiate the CROP of ε-CL monomer as depicted in Scheme 1. After polymerization, the resulted UCNP-g-PCL product was washed repeatedly with DCM to remove the ungrafted PCL (also called free PCL). To probe whether the content of PCL can be adjusted or not, UCNP-g-PCL with diverse values of weight feed ratio of ε-CL to UCNP-OH (Rwt) were fabricated by maintaining other parameters as constant (see Table 1). FTIR spectra of UCNP-g-PCL1 (Rwt = 8/1) and UCNP-g-PCL2 (Rwt = 20/1) show that the intensity of absorption bands at 2945, 2858, 1468, and 1160 cm−1 are markedly enhanced compared with UCNP-OH (Fig. 2a and S1†). Meanwhile, a new strong peak at 1726 cm−1 corresponding to O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O stretching vibration, and two obvious peaks at 1370 and 1250 cm−1 associated with C–H deformation vibration appeared. These results imply that PCL components have been grafted from the surface of UCNP-OH. The contents of PCL calculated from the TGA curves for UCNP-g-PCL1 and UCNP-g-PCL2 are 8.3 and 16.5 wt% (Fig. 2b), respectively, indicating that the amounts of grafted PCL associated with the thickness of PCL shell can be controlled by manipulating the Rwt. To give direct evidence for the tunability of PCL shell, the morphology of UCNP-g-PCL samples were observed by TEM. As can be seen in Fig. 1b and c, the thickness of PCL shell for UCNP-g-PCL1 and UCNP-g-PCL2 are respectively 2 and 5 nm, suggesting that the thickness of PCL shell can be adjusted by tuning the Rwt. This thickness tunability is very crucial since only polymer shell with appropriate thickness is desired for specific applications. In addition, the molecular weight parameters of grafted PCLs were calculated according to the TGA results. As shown in Table 1, the number-average molecule weight (Mn,TGA) of the grafted PCL increases from 1390 to 3040 with increasing Rwt from 8/1 to 20/1, suggesting that the molecular weight of grafted PCL can also be tuned by adjusting the Rwt.
C–O stretching vibration, and two obvious peaks at 1370 and 1250 cm−1 associated with C–H deformation vibration appeared. These results imply that PCL components have been grafted from the surface of UCNP-OH. The contents of PCL calculated from the TGA curves for UCNP-g-PCL1 and UCNP-g-PCL2 are 8.3 and 16.5 wt% (Fig. 2b), respectively, indicating that the amounts of grafted PCL associated with the thickness of PCL shell can be controlled by manipulating the Rwt. To give direct evidence for the tunability of PCL shell, the morphology of UCNP-g-PCL samples were observed by TEM. As can be seen in Fig. 1b and c, the thickness of PCL shell for UCNP-g-PCL1 and UCNP-g-PCL2 are respectively 2 and 5 nm, suggesting that the thickness of PCL shell can be adjusted by tuning the Rwt. This thickness tunability is very crucial since only polymer shell with appropriate thickness is desired for specific applications. In addition, the molecular weight parameters of grafted PCLs were calculated according to the TGA results. As shown in Table 1, the number-average molecule weight (Mn,TGA) of the grafted PCL increases from 1390 to 3040 with increasing Rwt from 8/1 to 20/1, suggesting that the molecular weight of grafted PCL can also be tuned by adjusting the Rwt.
| Sample | Rwta | fwtb% | Mn,TGAc |
|---|---|---|---|
| a Weight feed ratio of glycidol to UCNP-OH.b Weight fraction of grafted PCL calculated from corresponding TGA data between 150–600 °C.c Average molecular weight of the grafted PCL calculated from TGA data: Mn,TGA = mgra/ngra = fwt/[(1 − fwt) × 0.065 × 10−3]; herein, 0.065 represents the concentration of initiating sites per gram of UCNP-OH (mmol g−1), mgra and ngra represents the mass and molar amount of the grafted PCL, respectively. | |||
| UCNP-g-PCL1 | 8/1 | 8.3 | 1390 |
| UCNP-g-PCL2 | 20/1 | 16.5 | 3040 |
As displayed in Fig. 3, both UCNP-g-PCL1 and UCNP-g-PCL2 in DCM (1 mg mL−1) exhibit strong upconversion luminescence under the illumination of 980 nm laser. Correspondingly, their upconversion luminescence spectra were measured and the results are presented in Fig. 3. Three typical emission peaks at 656, 542, and 524 nm associated with the 4F9/2 → 4I15/2, 4S3/2 → 4I15/2, and 2H11/2 → 4I15/2 transitions for Er3+, respectively, can be clearly seen.33,38 Benefiting from the introduction of hydrophobic PCL shell, the as-prepared UCNP-g-PCL nanohybrids exhibited excellent dispersibility in low-boiling organic solvents such as chloroform, DCM and tetrahydrofuran (THF) (Fig. S2†). Through simple blending of the UCNP-g-PCL2 with poly(methyl methacrylate) (PMMA) in DCM, followed by solvent evaporation, upconverting nanocomposite film of UCNP-g-PCL/PMMA (the thickness is 0.48 mm) was obtained. The UCNP-g-PCL/PMMA film with 1 wt% of UCNP-g-PCL2 is almost colorless and highly transparent as demonstrated by the UV-vis transmittance spectra (Fig. 4a). The high transparence of the UCNP-g-PCL/PMMA film is attributed to the well dispersion of UCNP-g-PCL2 nanohybirds in the PMMA matrix as demonstrated by the TEM image in Fig. S3.† Compared with the UCNP-g-PCL2 in DCM (Fig. 3), no obvious changes in the upconversion luminescence spectrum (Fig. 4b) of UCNP-g-PCL/PMMA film could be found, implying that the structure and surface of the UCNP-g-PCL2 were well retained during the fabrication process.
3.2 Synthesis and characterization of UCNP-g-HPG
To demonstrate the robustness of surface-initiated CROP technique, in situ growth of multihydroxy HPG, a well-known biocompatible and water-soluble polymer, on the surface of UCNP was also performed by the CROP of glycidol monomer at low temperature (−20 °C).39–43 The successful grafting of HPG was confirmed by the TGA, FTIR and TEM measurements. Obvious weight-loss for UCNP-g-HPG sample was noticed between 150–600 °C (Fig. 5a), which was attributed to the thermal degradation of HPG polymer on the surface of UCNP. According to the TGA curve, the amount of grafted HPG was estimated to be 18.3 wt%. Compared with the FTIR result of UNCP-OH, the characteristic absorption bands of C–H at 2945, 2858, and 1468 cm−1, C–O at 1050–1160 cm−1, and O–H at 3450 cm−1 were enhanced significantly for UCNP-g-HPG due to the existence of HPG component (Fig. 5b). In addition, two obvious peaks at 1378 and 1250 cm−1 corresponding to C–H deformation vibration appeared. From the TEM image of UCNP-g-HPG (Fig. 5c), the HPG shell can be clearly observed on the surface of UCNP, further confirmed the successful grafting of HPG. Moreover, the UCNP-g-HPG still exhibited strong upconversion luminescence (Fig. 5d and S2†), suggesting that the CROP process did not affect the upconversion luminescence property of UCNP. The obtained UCNP-g-HPG displayed superior dispersibility in water and polar organic solvents, such as methanol, ethanol, and DMF. To further examine the colloidal stability of UCNP-g-HPG nanohybrids, their hydrodynamic sizes were determined by dynamic light scattering (DLS) measurements. The DLS result indicated that the average diameter of UCNP-g-HPG in water is larger than the result from TEM image (Fig. S4†). This is due to the HPG macromolecular chains were outspread in water while highly shrunk after drying on copper grids. After being stored for 12 h, the aqueous dispersion of UCNP-g-HPG displayed much higher colloidal stability than that of UCNP-OH due to the introduction of water-soluble HPG shell. This nice solution stability paves the way for further applications of UCNP-g-HPG in bioimaging, sensing, and light-emitting devices. All these results provided strong support that the surface-initiated CROP technique is a robust tool for grafting of polymers from the surface of UCNP.Next, the cytotoxicity of UCNP-g-HPG nanohybrids was assessed for NIH-3T3 mouse embryo fibroblast cells using a standard MTT cell-viability assay. As presented in Fig. 6a, the cell viabilities approached 100% when the concentration of UCNP-g-HPG was lower than 100 μg mL−1 and remained higher than 90% even when the concentration reached 500 μg mL−1 within the tested period, implying low cytotoxicity of the UCNP-g-HPG. On the basis of the outstanding water-dispersibility, strong upconversion luminescence and low cytotoxicity of UCNP-g-HPG, the use of UCNP-g-HPG for cellular imaging was investigated. MCF-7 breast cancer cells were incubated with UCNP-g-HPG solution (50 μg mL−1) and the luminescence imaging of cells was recorded by CLSM. The excitation wavelength was fixed at 980 nm. The CLSM images of UCNP-g-HPG stained MCF-7 cells is shown in Fig. 6b and c. Strong upconversion luminescence from the cellular cytoplasm is seen for MCF-7 cells, indicating that UCNP-g-HPG nanohybrids were efficiently internalized by the MCF-7 cells and accumulated in the cytoplasm. This result suggests that UCNP-g-HPG can be utilized as an effective upconverting luminescence stain for cell imaging with good luminescent contrast.
As various functional molecules are needed to conjugate with imaging agent for advanced bioapplications, it is necessary for a luminescent agent to have multiple reactive functional groups.44 As the UCNP-g-HPG nanohybrid possesses abundant hydroxyl groups, it can provide a convenient reaction platform for further conjugation and release of specific drug molecules. Herein, fluorescent dye RB was selected as a model drug molecule to investigate the drug delivery properties of the UCNP-g-HPG. The RB molecule was bound with UCNP-g-HPG through an easy Steglish esterification reaction between the hydroxyl groups of UCNP-g-HPG and the carboxylic groups of RB molecules (Scheme 1). The reaction process was studied by FTIR spectra as depicted in Fig. 5b. Compared with UCNP-g-HPG, the resulted UCNP-g-HPG-RB present a obvious new peak at 1726 cm−1 associated with the ester linkage (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), demonstrating the successful post-functionalization of UCNP-g-HPG. In addition, the aqueous dispersion of UCNP-g-HPG-RB could exhibit simultaneously upconversion luminescence (Fig. 5d) and downconversion luminescence (Fig. S5†). It is worth noting that the ratio of the upconversion luminescence intensity at 656 to 542 nm (I656/I542) for UCNP-g-HPG-RB (ca. 0.55) is much higher than that of UCNP-g-HPG (ca. 0.43) due to the presence of fluorescence resonance energy transfer (FRET) (Fig. 5d and S6a†). Similar phenomenon was also observed by other group.45 On one hand, the UCNP served as a donor and part of the emission light at the upconversion luminescence peak of 542 nm was absorbed by the RB moieties because the RB molecules possess a strong absorption band at around 562 nm (Fig. 7a). On the other hand, the RB acceptor can emit a fluorescence peak at around 587 nm after absorbing upconversion luminescence of the UCNP at 542 nm as shown in Fig S6b.†
O), demonstrating the successful post-functionalization of UCNP-g-HPG. In addition, the aqueous dispersion of UCNP-g-HPG-RB could exhibit simultaneously upconversion luminescence (Fig. 5d) and downconversion luminescence (Fig. S5†). It is worth noting that the ratio of the upconversion luminescence intensity at 656 to 542 nm (I656/I542) for UCNP-g-HPG-RB (ca. 0.55) is much higher than that of UCNP-g-HPG (ca. 0.43) due to the presence of fluorescence resonance energy transfer (FRET) (Fig. 5d and S6a†). Similar phenomenon was also observed by other group.45 On one hand, the UCNP served as a donor and part of the emission light at the upconversion luminescence peak of 542 nm was absorbed by the RB moieties because the RB molecules possess a strong absorption band at around 562 nm (Fig. 7a). On the other hand, the RB acceptor can emit a fluorescence peak at around 587 nm after absorbing upconversion luminescence of the UCNP at 542 nm as shown in Fig S6b.†
The release of RB molecules from the UCNP-g-HPG-RB solution was carried out by adding lipase (Scheme 2), which can cause the cleavage of ester linkage.46 For comparison, a control experiment was conducted, in which no lipase was added into the UCNP-g-HPG-RB solution. At predetermined intervals, the release medium was collected by centrifugation for analysis. As depicted in Fig. 7a, the absorbance value of the supernatant at 562 nm associated with the amount of RB gradually increased with increasing release time, demonstrating the successful release of RB component from the UCNP-g-HPG-RB. The release process could also be detected by naked eyes since the bright rose color of release medium gradually enhanced (Fig. 7b). On the contrary, no detectable color was observed for the supernatant of UCNP-g-HPG-RB solution without addition of lipase, indicating that the release of RB was attributed to the cleavage of O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds by lipase. Therefore, the UCNP-g-HPG nanohybrids not only possess strong upconversion luminescence, excellent solution dispersibility, and favorable biocompatibility, but also can serve as a versatile platform for further conjugation and release of desired functional molecules to meet various applications. On the basis of the above results, it is concluded that the surface-initiated CROP technique can be employed as a robust tool to tailor the surface properties of UCNPs by grafting diverse polymers from their surfaces.
O bonds by lipase. Therefore, the UCNP-g-HPG nanohybrids not only possess strong upconversion luminescence, excellent solution dispersibility, and favorable biocompatibility, but also can serve as a versatile platform for further conjugation and release of desired functional molecules to meet various applications. On the basis of the above results, it is concluded that the surface-initiated CROP technique can be employed as a robust tool to tailor the surface properties of UCNPs by grafting diverse polymers from their surfaces.
4 Conclusions
In conclusion, we have demonstrated an easy and effective procedure to tailor the surface of UCNP with covalently linked polymers via surface-initiated cationic ring-opening polymerization. This approach allows for controllable tuning of the amounts of grafted polymers by adjusting the feed ratio. The polymerization process has no obvious quenching effect on the upconversion luminescence property of the UCNPs. Furthermore, the grafted polymers can not only enhance the solution dispersibility and processibility of the UCNPs but also endow the UCNPs with high biocompatibility and favorable surface functionality, as confirmed by the fabrication of transparent upconverting UCNP-g-PCL/PMMA film, and MTT and lipase-induced drug delivery results. In a word, this work presents the first example of UCNP/polymer nanohybrids prepared by CROP, which not only opens up new opportunities for altering surface chemistry of UCNPs, but also expands the application fields of UNCPs.Acknowledgements
The authors appreciate financial support from the National Natural Science Foundation of China (No. 51103028 and No. 21364003), Guangxi Natural Science Foundation (Grant No. 2014GXNSFCA118004), and project of outstanding young teachers' training in higher education institutions of Guangxi.Notes and references
- F. Wang, Y. Han, C. S. Lim, Y. H. Lu, J. Wang, J. Xu, H. Y. Chen, C. Zhang, M. H. Hong and X. G. Liu, Nature, 2010, 463, 1061–1065 CrossRef CAS PubMed.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- M. Wang, G. Abbineni, A. Clevenger, C. B. Mao and S. K. Xu, J. Nanomed. Nanotechnol., 2011, 7, 710–729 CrossRef CAS PubMed.
- W. Feng, C. M. Han and F. Y. Li, Adv. Mater., 2013, 25, 5287–5303 CrossRef CAS PubMed.
- D. Yang, P. Ma, Z. Hou, Z. Cheng, C. Li and J. Lin, Chem. Soc. Rev., 2015, 44, 1416–1448 RSC.
- N. M. Idris, M. K. G. Jayakumar, A. Bansal and Y. Zhang, Chem. Soc. Rev., 2015, 44, 1449–1478 RSC.
- Y. Sun, W. Feng, P. Yang, C. Huang and F. Li, Chem. Soc. Rev., 2015, 44, 1509–1525 RSC.
- L. Zhou, Z. Chen, K. Dong, M. Yin, J. Ren and X. Qu, Adv. Mater., 2013, 26, 2424–2430 CrossRef PubMed.
- G. S. Yi and G. M. Chow, Chem. Mater., 2007, 19, 341–343 CrossRef CAS.
- S. Yang, N. Li, Z. Liu, W. Sha, D. Chen, Q. Xu and J. Lu, Nanoscale, 2014, 6, 14903–14910 RSC.
- V. Muhr, S. Wilhelm, T. Hirsch and O. S. Wolfbeis, Acc. Chem. Res., 2014, 47, 3481–3493 CrossRef CAS PubMed.
- M. Liras, M. González-Béjar, E. Peinado, L. Francés-Soriano, J. Pérez-Prieto, I. Quijada-Garrido and O. García, Chem. Mater., 2014, 26, 4014–4022 CrossRef CAS.
- F. Wang, D. K. Chatterjee, Z. Li, Y. Zhang, X. Fan and M. Wang, Nanotechnology, 2006, 17, 5786–5791 CrossRef CAS.
- Z. Li and Y. Zhang, Angew. Chem., Int. Ed., 2006, 45, 7732–7735 CrossRef CAS PubMed.
- T. Y. Cao, Y. Yang, Y. A. Gao, J. Zhou, Z. Q. Li and F. Y. Li, Biomaterials, 2011, 32, 2959–2968 CrossRef CAS PubMed.
- J. F. Jin, Y. J. Gu, C. W. Y. Man, J. P. Cheng, Z. H. Xu, Y. Zhang, H. S. Wang, V. H. Y. Lee, S. H. Cheng and W. T. Wong, ACS Nano, 2011, 5, 7838–7847 CrossRef CAS PubMed.
- R. Naccache, F. Vetrone, V. Mahalingam, L. A. Cuccia and J. A. Capobianco, Chem. Mater., 2009, 21, 717–723 CrossRef CAS.
- P. Cao, L. Tong, Y. Hou, G. Zhao, G. Guerin, M. A. Winnik and M. Nitz, Langmuir, 2012, 28, 12861–12870 CrossRef CAS PubMed.
- S. J. Budijono, J. N. Shan, N. Yao, Y. Miura, T. Hoye, R. H. Austin, Y. G. Ju and R. K. Prud'homme, Chem. Mater., 2010, 22, 311–318 CrossRef CAS.
- L. He, L. Feng, L. Cheng, Y. Liu, Z. Li, R. Peng, Y. Li, L. Guo and Z. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 10381–10388 CAS.
- Q. A. Liu, C. Y. Li, T. S. Yang, T. Yi and F. Y. Li, Chem. Commun., 2010, 46, 5551–5553 RSC.
- Q. Zhang, K. Song, J. Zhao, X. Kong, Y. Sun, X. Liu, Y. Zhang, Q. Zeng and H. Zhang, J. Colloid Interface Sci., 2009, 336, 171–175 CrossRef CAS PubMed.
- C. Wang, H. Tao, C. Liang and Z. Liu, Biomaterials, 2011, 32, 6145–6154 CrossRef CAS PubMed.
- G. Zhao, L. Tong, P. Cao, M. Nitz and M. A. Winnik, Langmuir, 2014, 30, 6980–6989 CrossRef CAS PubMed.
- L. Wang, R. Yan, Z. Huo, L. Wang, J. Zeng, J. Bao, X. Wang, Q. Peng and Y. Li, Angew. Chem., Int. Ed., 2005, 44, 6054–6057 CrossRef CAS PubMed.
- H. Kong, C. Gao and D. Yan, J. Am. Chem. Soc., 2004, 126, 412–413 CrossRef CAS PubMed.
- L. Q. Xu, D. Wan, H. F. Gong, K.-G. Neoh, E.-T. Kang and G. D. Fu, Langmuir, 2010, 26, 15376–15382 CrossRef CAS PubMed.
- L. Zhou, C. Gao and W. Xu, Macromol. Chem. Phys., 2009, 210, 1011–1018 CrossRef CAS.
- Q. Feng, D. Tang, H. Lv, W. Zhang and W. Li, RSC Adv., 2015, 5, 62024–62032 RSC.
- L. Zhou, C. Gao and W. Xu, J. Mater. Chem., 2009, 19, 5655–5664 RSC.
- H. Hua, Y. Xiong, C. Fu and N. Li, RSC Adv., 2014, 4, 39273–39279 RSC.
- L. Zhou, B. He, J. Huang, Z. Cheng, X. Xu and C. Wei, ACS Appl. Mater. Interfaces, 2014, 6, 7719–7727 CAS.
- B. He, L. Zhou and J. Huang, Mater. Lett., 2014, 117, 142–145 CrossRef CAS.
- M. A. Tavares Cardoso, M. Talebi, P. A. M. H. Soares, C. U. Yurteri and J. R. van Ommen, Int. J. Pharm., 2011, 414, 1–23 CrossRef CAS PubMed.
- H. Zeng, C. Gao and D. Yan, Adv. Funct. Mater., 2006, 16, 812–818 CrossRef CAS.
- G. Carrot, D. Rutot-Houzé, A. Pottier, P. Degée, J. Hilborn and P. Dubois, Macromolecules, 2002, 35, 8400–8404 CrossRef CAS.
- L. Zhou, C. Gao, D. Zhu, W. Xu, F. F. Chen, A. Palkar, L. Echegoyen and E. S. W. Kong, Chem.–Eur. J., 2009, 15, 1389–1396 CrossRef CAS PubMed.
- S. Gai, C. Li, P. Yang and J. Lin, Chem. Rev., 2014, 114, 2343–2389 CrossRef CAS PubMed.
- R. K. Kainthan, S. R. Hester, E. Levina, D. V. Devine and D. E. Brooks, Biomaterials, 2007, 28, 4581–4590 CrossRef CAS PubMed.
- M. Calderón, M. A. Quadir, S. K. Sharma and R. Haag, Adv. Mater., 2010, 22, 190–218 CrossRef PubMed.
- L. Zhou, J. Geng, G. Wang, J. Liu and B. Liu, ACS Macro Lett., 2012, 1, 927–932 CrossRef CAS.
- H. Cheng, S. Wang, J. Yang, Y. Zhou and D. Yan, J. Colloid Interface Sci., 2009, 337, 278–284 CrossRef CAS PubMed.
- L. Zhou, J. Geng, G. Wang, J. Liu and B. Liu, Polym. Chem., 2013, 4, 5243–5251 RSC.
- G. Y. Chen, H. L. Qiu, P. N. Prasad and X. Y. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed.
- M. González-Béjar, M. Liras, L. Francés-Soriano, V. Voliani, V. Herranz-Pérez, M. Duran-Moreno, J. M. Garcia-Verdugo, E. L. Alarcon, J. C. Scaiano and J. Pérez-Prieto, J. Mater. Chem. B, 2014, 2, 4554–4563 RSC.
- M. H. Xiong, Y. Bao, X. Z. Yang, Y. C. Wang, B. Sun and J. Wang, J. Am. Chem. Soc., 2012, 134, 4355–4362 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR spectrum of UCNP-OH, photographs of UCNP-g-PCL2 and UCNP-g-HPG in the mixture of water and chloroform under daylight and 980 nm laser illumination, TEM image of UCNP-g-PCL/PMMA film, hydrodynamic radius distribution of aqueous dispersion of UCNP-OH and UCNP-g-HPG for different time, downconversion luminescence spectrum of UCNP-g-HPG-RB, and more upconversion emission spectra of UCNP-g-HPG and UCNP-g-HPG-RB. See DOI: 10.1039/c5ra18922e |
| This journal is © The Royal Society of Chemistry 2015 |