Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers – a review
Abstract
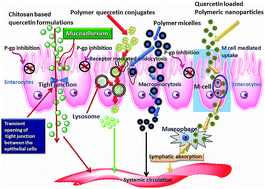
With numerous pharmacological and biological functions bio-flavonoids gain appreciable attention in diabetes and other therapeutic research. Among several beneficial flavonoids quercetin exhibits impressive hypoglycemic effects, with significant improvement, stabilization of long sustaining insulin secretion and regeneration of human islets in the pancreas without producing serious health hazards. However, in oral delivery poor solubility, stability in biological milieu, low permeation, short biological half-life, and insignificant bioavailability limit its wide application in anti diabetic research. Over the last few decades polymeric carrier systems have been widely studied for improvement of quercetin bioavailability. Natural polymers are more preferred in this regard as they possess several favourable properties like biocompatibility, biodegradability, mucoadhesiveness, non-immunogenicity and non-toxicity. This review focuses on quercetin in anti-diabetic research and the progress in the synthesis of polymer-based formulations for efficient quercetin delivery, with an emphasis on producing an improved biological efficacy of the flavonoid. Diabetic complications, probable mechanisms of quercetin absorption, regulation and anti diabetic effects, obstacles to produce desired bio-efficacy and possible remedies are also brought into focus. To overcome these barriers encapsulation of quercetin within various safe polymeric vehicles are discussed. Further, this review sheds light on enhancing the efficacy of quercetin in novel ways for successful diabetes treatment and others.

 Please wait while we load your content...
Please wait while we load your content...