First direct access to 2-hydroxybenzophenones via nickel-catalyzed cross-coupling of 2-hydroxybenzaldehydes with aryl iodides†
N. Nowrouzi*,
M. Zarei and
F. Roozbin
Department of Chemistry, Faculty of Sciences, Persian Gulf University, Bushehr, 75169, Iran. E-mail: nowrouzi@pgu.ac.ir
First published on 24th November 2015
Abstract
An efficient, inexpensive and reusable NiCl2·6H2O/n-Bu4NBr catalytic system is described for the direct C–H arylation of 2-hydroxybenzaldehydes with aryl iodides for the first time.
Aryl ketones are valuable building blocks in both chemistry and biology that have been widely used in the pharmaceutical, fragrance, dye, agrochemical, and functional material industries as well as in organic synthesis.1
Friedel–Crafts acylation in the presence of Lewis acids is one of the most useful synthetic methods for the preparation of aromatic ketones, although the reaction sometimes fails with electron-deficient arenes and generally yields the desired products as isomeric mixtures. In addition, it requires the use of a stoichiometric amount of Lewis acid.2 Oxidation of secondary alcohols is another common method to access ketones. This protocol often requires the use of a stoichiometric amount of oxidant and is commonly limited by poor functional group tolerance.3 The reactions of stoichiometric organometallic compounds with carboxylic acid derivatives are also popular and well-known for the preparation of ketones.4 However, the poor functional group tolerance, strict handling requirement of the organometallic reagents and overaddition (resulting in the formation of tertiary alcohol products) limits their applications and thus there is a need for milder, cleaner and catalytic alternatives. During the last decade, transition-metal catalyzed cross-coupling reactions have emerged as powerful methods in synthetic chemistry. Among many methods, direct C–H bond arylation of aldehydes and their derivatives to give aryl ketones has attracted a lot of attention.5 However, in some of these transformations the existence of a hydroxyl group in the substrates led to main impediment. The prevalence of ortho-hydroxyl-substituted diaryl ketones in a large number of biologically active compounds,6 in perfumery, pharmaceutical industries and also natural products,7 has resulted in a continued demand for the development of general and flexible synthetic methods of this structural moiety. Generally, reports on the direct access to 2-hydroxybenzophenones from 2-hydroxyarylaldehydes via metal-catalyzed cross-coupling reactions are very rare in the literature. The first palladium-catalyzed reaction of 2-hydroxybenzaldehyde and its derivatives with aryl iodides via cleavage of the aldehyde C–H bond to obtain the corresponding ketones has been reported by Miura in 1996.8 This transformation gives good yields for many substrates using a catalyst system of PdCl2/LiCl in the presence of Na2CO3 as base in DMF at 100 °C under nitrogen. Several years later, effort has been directed towards the preparation of 2-hydroxybenzophenones using iodonium salts as analogues of aryl iodides.9 These salts were used into the reaction with salicylaldehyde in the presence of PdCl2 and LiCl as a co-catalyst in DMF to achieve corresponding ketones. In 2010, Xu reported an efficient system for the direct arylation of 2-hydroxybenzaldehydes with arylboronic acids via ligand-free palladium catalysis.10 However, the boronic acids are comparably expensive and not so widely available. Most recently, we have reported an efficient method for the palladium-catalyzed cross-coupling reaction of aryl bromides and iodides with 2-hydroxybenzaldehydes in H2O to access 2-hydroxybenzophenones without the need for any co-catalyst and ligand.11
Whiles palladium based complexes are the most common catalysts for the coupling reactions, efforts have been made to replace palladium with less expensive metals. Among the inexpensive transition metals, Ni appears the most promising for the replacement of Pd because it is active for coupling reactions and about 500 times cheaper than palladium.
Despite the fact that various nickel-catalyzed systems are available in the literature for the synthesis of aryl ketones,12 to the best of our knowledge there is no literature precedent for the use of nickel for the preparation of 2-hydroxybenzophenones using 2-hydroxybenzaldehydes. Herein we wish to report a direct synthesis of 2-hydroxybenzophenones by the nickel-catalyzed coupling of 2-hydroxybenzaldehydes with aryl halides via an oxidative addition and reductive elimination strategy in a one-pot manner.
We began our study by examining the cross-coupling between iodobenzene (1.0 mmol) and 2-hydroxybenzaldehyde (1.0 mmol) using 50 mol% NiCl2·6H2O as catalyst in ethylene glycol (2 mL) at 120 °C in the presence of NaOH (2.0 mmol) as base to furnish the corresponding diaryl ketone. Table 1 provides information on the impact of various reaction parameters on the efficiency of this process. Surprisingly, we discovered that even without any reducing agent and ligand, the reaction proceeded smoothly, affording the coupling product after 24 h, albeit in low yield (Table 1, entry 1). This promising result encouraged us to do further optimization with NiCl2·6H2O as the catalyst precursor to improve the yield of the product. Solvent screening showed that no product could be detected when the reaction was carried out in ethanol, dioxane, and H2O at reflux (Table 1, entries 2–4). Also, no desired product was obtained in PEG at 120 °C (Table 1, entry 5). Therefore, the subsequent reactions were performed in ethylene glycol which possesses negligible vapor pressure, is thermally stable and inexpensive. We surveyed several different organic and inorganic bases for the coupling reaction, and NaOH was found to give the best results (Table 1, entry 1). Absence of base, or changing the base to Na2CO3, Bu3N and DABCO did not yield the target product at all (entries 6–9). In a further attempt to improve the yield of aryl ketone, the use of 2.0 mmol of iodobenzene was explored (Table 1, entry 10). Gratifyingly, this change afforded higher yield of the product (from 40% to 75%). No further yield improvement was achieved using greater amounts of iodobenzene (Table 1, entry 11). It was interesting to find that the significant improvement in the reaction time and the yield of 2-hydroxybenzophenone was observed following the addition of 0.5 mmol tetrabutylammonium bromide to the reaction mixture (Table 1, entry 12). The role of TBAB in this reaction is critical. It is noteworthy that the presence of TBAB as an additive not only makes the catalytic system stable by preventing undesired agglomeration and deactivation by forming a monomolecular layer around the metal core,13 but also acts as reducing agent for the generation of Ni(0). Next, the reaction was carried out with different amounts of NiCl2·6H2O (30, 40, 60 mol%). As it was shown in entries 13–15 of Table 1, at 120 °C, 40 mol% of the catalyst was sufficient to catalyze the reaction efficiently; in this case, the corresponding product was obtained in 94% yield within 2 h (Table 1, entry 14). As expected, no product could be detected in the absence of nickel catalyst (Table 1, entry 16). A temperature of 120 °C was the optimal temperature for this reaction. Decreasing the reaction temperature caused a decrease in the yield of desired product (Table 1, entry 17) and higher temperature (Table 1, entry 18) did not improve the conversion and yield. Next, the model reaction was carried out in the absence of ethylene glycol. The result indicated that the existence of ethylene glycol is necessary in this transformation (Table 1, entry 19). Finally, we decided to re-examine the coupling reaction of iodobenzene and salicylaldehyde using NiCl2 with a purity of ≥99.99% instead of nickel salt with a purity of ≥98% under our optimized reaction conditions to confirm that the reaction was not catalyzed by a trace metal contaminant. As shown in Table 1, entry 20, the use of NiCl2 with a purity of ≥99.99% did not led to lower yield than the same reaction with nickel salt of lower purity. This observation indicated that the presence of trace amounts of metal impurities in the commercially available nickel catalyst were not capable of catalyzing the coupling reactions.
| Entry | Catalysta (mol%) | Base | Solvent/°C | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a NiCl2·6H2O ≥ 98% purity was used.b Isolated yield.c 2.0 mmol iodobenzene was used.d 3.0 mmol iodobenzene was used.e NiCl2 ≥ 99.99% purity was used. | |||||
| 1 | 50 | NaOH | Ethylene glycol 120 | 24 | 40 |
| 2 | 50 | NaOH | EtOH/reflux | 24 | — |
| 3 | 50 | NaOH | Dioxane/reflux | 24 | — |
| 4 | 50 | NaOH | H2O/reflux | 24 | — |
| 5 | 50 | NaOH | PEG/120 | 24 | — |
| 6 | 50 | — | Ethylene glycol 120 | 24 | — |
| 7 | 50 | Na2CO3 | Ethylene glycol 120 | 24 | — |
| 8 | 50 | Bu3N | Ethylene glycol 120 | 24 | — |
| 9 | 50 | DABCO | Ethylene glycol 120 | 24 | — |
| 10c | 50 | NaOH | Ethylene glycol 120 | 24 | 75 |
| 11d | 50 | NaOH | Ethylene glycol 120 | 24 | 75 |
| 12c | 50 | NaOH | Ethylene glycol TBAB/120 | 2 | 94 |
| 13c | 60 | NaOH | Ethylene glycol TBAB/120 | 2 | 93 |
| 14c | 40 | NaOH | Ethylene glycol TBAB/120 | 2 | 94 |
| 15c | 30 | NaOH | Ethylene glycol TBAB/120 | 9 | 75 |
| 16c | — | NaOH | Ethylene glycol TBAB/120 | 24 | — |
| 17c | 40 | NaOH | Ethylene glycol TBAB/100 | 24 | 70 |
| 18c | 40 | NaOH | Ethylene glycol TBAB/140 | 2 | 94 |
| 19c | 40 | NaOH | 120/TBAB | 24 | Trace |
| 20c,e | 40 | NaOH | Ethylene glycol TBAB/120 | 2 | 93 |
Under the obtained optimized conditions; NiCl2·6H2O (40 mol%), aryl halide (2.0 mmol), 2-hydroxybenzaldehyde (1.0 mmol), NaOH (2.0 mmol) and tetrabutylammonium bromide (0.5 mmol) in ethylene glycol (2 mL) at 120 °C, the substrate scope of the direct arylation of 2-hydroxybenzaldehydes was explored. As shown by the results in Table 2, this cross coupling is efficiently applicable to a wide range of aryl iodides. The coupling reaction with electron-neutral, electron-rich and electron-poor aryl iodides were carried out successfully at 120 °C to generate the corresponding products in good to excellent yields. The influence of steric effects was studied by introduction of an alkyl substituent into the ortho-position of the halobenzene ring. As shown by the data in entry 4 of Table 2, a substituent such as methyl at the ortho position had steric effect and in this case, no desired product was obtained even after prolonged reaction time. We then studied the applicability of this catalytic system for the reaction of aryl bromides. For this purpose, reaction of bromobenzene with salicylaldehyde under the optimal conditions was studied. The data presented in entry 12 of Table 2 show that, the reaction did not proceed at all and starting material was isolated intact after appropriate reaction time. Attempts to find reaction conditions that enabled the conversion of bromobenzene to product, such as increasing the concentration of bromobenzene or the addition of more nickel catalyst during the reaction, failed. Also, the addition of PPh3 which is often necessary for the Pd-catalyzed coupling reactions, did not improve the conversion and yield of the coupling product.
| Entry | Aldehyde | ArX | Time (h) | Yieldb (%) (ref) |
|---|---|---|---|---|
| a Reaction conditions: iodobenzene (2.0 mmol), 2-hydroxybenzaldehyde (1.0 mmol), NiCl2·6H2O (40 mol%), n-Bu4NBr (0.5 mmol), NaOH (2.0 mmol) in 2 mL ethylene glycol at 120 °C.b Isolated yield. | ||||
| 1 | 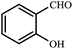 |
 |
2 | 94 (ref. 9) |
| 2 |  |
 |
8 | 90 (ref. 14) |
| 3 |  |
 |
16 | 83 (ref. 9) |
| 4 |  |
 |
48 | — |
| 5 |  |
 |
24 | 60 (ref. 15) |
| 6 |  |
 |
18 | 78 (ref. 16) |
| 7 |  |
 |
20 | 80 (ref. 16) |
| 8 |  |
 |
8 | 88 (ref. 17) |
| 9 |  |
 |
6 | 89 (ref. 18) |
| 10 |  |
 |
24 | 70 (ref. 19) |
| 11 |  |
 |
24 | 75 (ref. 9) |
| 12 |  |
 |
24 | — |
| 13 |  |
 |
24 | — |
| 14 |  |
 |
24 | — |
To explore the reaction mechanism, the following control experiments were performed under the standard reaction conditions. Coupling of p-hydroxybenzaldehyde with iodobenzene gave no desired 4-hydroxybenzophenone (Table 2, entry 13). Furthermore, the reaction of benzaldehyde with iodobenzene under the optimal conditions yielded no benzophenone (Table 2, entry 14). These results suggested that the phenolic function at the ortho-position of the formyl group is essential for successful performance of the reaction.
In order to gain insight about the reducing property of ethylene glycol and tetrabutylammonium bromide, we probed the ultraviolet (UV) spectrum of Ni(II) in the presence of TBAB and ethylene glycol separately. The UV spectra of these solutions are shown in Fig. 1. The peak at around 400 and 700 nm of curve A shows the presence of Ni(II) species. Curves B and C belong to the solution of NiCl2·6H2O in ethylene glycol and TBAB respectively. The disappearance of the peaks related to Ni(II) at 400 and 700 nm, confirms that Ni(II) has been reduced to the Ni(0) species in the presence of ethylene glycol and TBAB (Fig. 1).20–22
 | ||
| Fig. 1 UV spectra of the nickel catalyst. (A) NiCl2·6H2O in H2O. (B) NiCl2·6H2O in ethylene glycol. (C) NiCl2·6H2O in TBAB. | ||
According to the above results, a proposed mechanism for the aryl ketone formation is shown in Scheme 1. Step 1 involves an oxidative addition in which Ni(0) inserts into the aryl halide bond. The formed nickel(II) species subsequently reacts with salicylaldehyde to give aryl(aryloxy)nickel intermediate I. Insertion of the aryl to the carbonyl group gives intermediate II, which then gives intermediate III via a β-hydride elimination process. Finally, the subsequent reductive elimination from III, yields 2-hydroxybenzophenone and also regenerates the nickel(0) catalyst.
The reusability of catalyst is the major area of interest due to environmental and economic concerns. One of the significant advantages of the use of ammonium salts as reaction medium is possibility of catalyst recycling. Regarding this property of ammonium salts, the recyclability of the catalytic system was also examined in the reaction of iodobenzene with salicylaldehyde. After initial experimentation, the reaction mixture was extracted with diethyl ether, and the obtained Ni(0)/n-Bu4NBr/ethylene glycol was subjected to a second run of the reaction by charging with the same substrates (iodobenzene and salicylaldehyde). As shown in Table 3, quantitative conversion to the corresponding product was observed for three runs. From the fourth run, loss of the activity of the system was observed.
In summary, We have reported the first Ni(0) catalytic method for the efficient cross-coupling reaction of aryl iodides with 2-hydroxybenzaldehydes in the presence of n-Bu4NBr in ethylene glycol to access 2-hydroxybenzophenones.23 The absence of ligand, co-catalyst and external reducing agent in the catalytic system together with its recyclability are considered as other advantages of this system.
Acknowledgements
We thank the Persian Gulf University Research Council and Iran National Science Foundation (INSF-Grant number of 92012390) for support of this study.Notes and references
- (a) Y. Shen and C. F. Chen, Chem. Rev., 2012, 112, 1463 CrossRef CAS PubMed; (b) Y. B. Wu, Z. Y. Ni, Q. W. Shi, M. Dong, H. Kiyota, Y. C. Gu and B. Cong, Chem. Rev., 2012, 112, 5967 CrossRef CAS PubMed; (c) B. Skillinghaug, C. Skold, J. Rydfjord, F. Svensson, M. Behrends, J. Savmarker, P. J. R. Sjoberg and M. Larhed, J. Org. Chem., 2014, 79, 12018 CrossRef CAS PubMed; (d) X. Jia, S. Zhang, W. Wang, F. Luo and J. Cheng, Org. Lett., 2009, 11, 3120 CrossRef CAS PubMed; (e) K. R. Romines, G. A. Freeman, L. T. Schaller, J. R. Cowan, S. S. Gonzales, J. H. Tidwell, C. W. Andrews, D. K. Stammers, R. J. Hazen, R. G. Ferris, S. A. Short, J. H. Chan and L. R. Boone, J. Med. Chem., 2006, 49, 727 CrossRef CAS PubMed; (f) S. Rahimipour, C. Palivan, F. Barbosa, I. Bilkis, Y. Koch, L. Weiner, M. Fridkin, Y. Mazur and G. J. Gescheidt, J. Am. Chem. Soc., 2003, 125, 1376 CrossRef CAS PubMed; (g) S. Baggett, E. P. Mazzola and E. J. Kennelly, Stud. Nat. Prod. Chem., 2005, 32, 721 CAS.
- (a) G. A. Olah, Friedel–Crafts Chemistry, Wiley, New York, 1973 Search PubMed; (b) G. Sartori and R. Maggi, in Advances in Friedel–Crafts Acylation Reactions: Catalytic and Green Process, CRC Press, Florida, 2010 Search PubMed.
- (a) G. Tojo and M. Fernandez, Oxidation of Alcohols to Aldehyde and Ketones: A Guide to Current Common Practice, Springer, 2006 Search PubMed; (b) M. Hudlicky, in Oxidations in Organic Chemistry, ACS Monograph 186, American Chemical Society, Washington, DC, 1990 Search PubMed.
- (a) J. March, Advanced Organic Chemistry, Wiley, New York, 3rd edn, 1985, p. 433 and 824 Search PubMed; (b) R. K. Dieter, Tetrahedron, 1999, 55, 4177 CrossRef CAS.
- (a) L. Yang, T. Zeng, Q. Shuai, X. Guo and C. J. Li, Chem. Commun., 2011, 47, 2161 RSC; (b) J. Karthikeyan, K. Parthasarathy and C. H. Cheng, Chem. Commun., 2011, 47, 10461 RSC; (c) B. K. Dey, J. Dutta, M. G. B. Drew and S. Bhattacharya, J. Organomet. Chem., 2014, 750, 176 CrossRef CAS; (d) H. Rao, L. Yang, Q. Shuai and C. J. Li, Adv. Synth. Catal., 2011, 353, 1701 CrossRef CAS; (e) J. Y. Chen, S. C. Chen, Y. J. Tang, C. Y. Mou and F. Y. Tsai, J. Mol. Catal. A: Chem., 2009, 307, 88 CrossRef CAS; (f) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (g) M. Pucheault, S. Darses and J. P. Genet, J. Am. Chem. Soc., 2004, 126, 15356 CrossRef CAS PubMed; (h) Y. Fu, Y. Yang, H. M. Hügel, Z. Du, K. Wang, D. Huanga and Y. Hu, Org. Biomol. Chem., 2013, 11, 4429 RSC; (i) C. Qin, J. Chen, H. Wu, J. Cheng, Q. Zhang, B. Zuo, W. Su and J. Ding, Tetrahedron Lett., 2008, 49, 1884 CrossRef CAS; (j) J. R. Schmink and S. W. Krska, J. Am. Chem. Soc., 2011, 133, 19574 CrossRef CAS PubMed.
- (a) G. Tang, Z. Nikolovska-Coleska, S. Qiu, C. Yang, J. Guo and S. Wang, J. Med. Chem., 2008, 51, 717 CrossRef CAS PubMed; (b) S. Zhong, X. Chen, X. Zhu, B. Dziegielewska, K. E. Bachman, T. Ellenberger, J. D. Ballin, G. M. Wilson, A. E. Tomkinson and A. D. MacKerell Jr, J. Med. Chem., 2008, 51, 4553 CrossRef CAS PubMed; (c) T. J. Crisman, M. T. Sisay and J. Bajorath, J. Chem. Inf. Model., 2008, 48, 1955 CrossRef CAS PubMed.
- (a) A. Krick, S. Kehraus, C. Gerhuser, K. Klimo, M. Nieger, A. Maier, H. H. Fiebig, I. Atodiresei, G. Raabe, J. Fleischhauer and G. M. Knig, J. Nat. Prod., 2007, 70, 353 CrossRef CAS PubMed; (b) Y. Deng, Y. W. Chin, H. Chai, W. J. Keller and A. D. Kinghorn, J. Nat. Prod., 2007, 70, 2049 CrossRef CAS PubMed; (c) C. Zhang, J. G. Ondeyka, K. B. Herath, Z. Guan, J. Collado, G. Platas, F. Pelaez, P. S. Leavitt, A. Gurnett, B. Nare, P. Liberator and S. B. Singh, J. Nat. Prod., 2005, 68, 611 CrossRef CAS PubMed; (d) J. Li, Y. Jiang and P. F. Tu, J. Nat. Prod., 2005, 68, 1802 CrossRef CAS PubMed; (e) M. Pecchio, P. N. Sols, J. L. Lpez-Prez, Y. Vsquez, N. Rodrguez, D. Olmedo, M. Correa, A. S. Feliciano and M. P. Gupta, J. Nat. Prod., 2006, 69, 410 CrossRef CAS PubMed.
- T. Satoh, T. Itaya, M. Miura and M. Nomura, Chem. Lett., 1996, 823 CrossRef CAS.
- M. Xia and Z. Chen, Synth. Commun., 2000, 30, 531 CrossRef CAS.
- F. Weng, C. Wang and B. Xu, Tetrahedron Lett., 2010, 51, 2593 CrossRef CAS.
- N. Nowrouzi, S. Motevalli and D. Tarokh, J. Mol. Catal. A: Chem., 2015, 396, 224 CrossRef CAS.
- (a) L. J. Gu, C. Jin and H. T. Zhang, Chem.–Eur. J., 2015, 21, 8741 CrossRef CAS PubMed; (b) S. H. Kim and R. D. Rieke, Tetrahedron Lett., 2011, 52, 1523 CrossRef CAS; (c) Y. C. Wong, K. Parthasarathy and C. H. Cheng, Org. Lett., 2010, 12, 1736 CrossRef CAS PubMed; (d) F. Wu, W. Lu, Q. Qian, Q. Ren and H. Gong, Org. Lett., 2012, 14, 3044 CrossRef CAS PubMed; (e) N. Iranpoor, H. Firouzabadi and E. Etemadi Davan, J. Organomet. Chem., 2015, 794, 282 CrossRef CAS.
- M. T. Reetz and J. G. de Vries, Chem. Commun., 2004, 1559 RSC.
- C. Bandyopadhyay, K. R. Sur and H. K. Das, J. Chem. Res., 1999, 2561 Search PubMed , Miniprint.
- (a) T. Das, A. Chakraborty and A. Sarkar, Tetrahedron Lett., 2014, 55, 7198 CrossRef CAS; (b) D. J. Chen and Z. C. Chen, Synlett, 2000, 1175 CAS.
- S. K. Chittimalla, T. C. Chang, T. C. Liu, H. P. Hsieh and C. C. Liao, Tetrahedron, 2008, 64, 2586 CrossRef CAS.
- O. Kysel, R. Zahradník and B. Pakula, Collect. Czech. Chem. Commun., 1970, 35, 3020 CrossRef CAS.
- C. Zhou and R. C. Larock, J. Org. Chem., 2006, 71, 3551 CrossRef CAS PubMed.
- A. R. Katritzky, K. N. B. Le and P. P. Mohapatra, Synthesis, 2007, 3141 CrossRef CAS.
- N. Iranpoor, H. Firouzabadi, K. R. Moghadam and S. Motavalli, RSC Adv., 2014, 4, 55732 RSC.
- N. Nowrouzi and M. Zarei, Tetrahedron, 2015, 71, 7847 CrossRef CAS.
- S. V. Kryatov, B. S. Mohanraj, V. V. Tarasov, O. P. Kryatova, E. V. Rybak-Akimova, B. Nuthakki, J. F. Rusling, R. J. Staples and A. Y. Nazarenko, Inorg. Chem., 2002, 41, 923 CrossRef CAS PubMed.
- General procedure: a mixture of tetrabutylammonium bromide (0.5 mmol, 0.16 g), aryl iodide (2.0 mmol), 2-hydroxybenzaldehyde (1.0 mmol), NiCl2·6H2O (0.4 mmol, 0.114 g) and NaOH (2.0 mmol, 0.08 g) were added to a flask containing 2 mL of ethylene glycol. The mixture was heated in an oil bath at 120 °C with stirring and the reaction was followed by TLC analysis. When the reaction was completed, the solution was allowed to cool to room temperature and the product was extracted with diethyl ether (3 × 3 mL). The organic layer was dried over anhydrous Na2SO4 and purified by column chromatography over silica gel using n-hexane/ethyl acetate (5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to afford the highly pure product (Table 2). 2-Hydroxybenzophenone9 (Table 2, entry 1) [117–99–7]: IR (KBr): 3500, 1728 cm−1. 1H-NMR (250 MHz, CDCl3) δ (ppm): 11.96 (s, 1H, OH), 7.62–7.40 (m, 7H, Ar), 7.02–6.98 (m, 1H, Ar), 6.83–6.77 (m, 2H, Ar). 13C-NMR (62.9 MHz, CDCl3) δ (ppm): 200.01, 158.02, 140.77, 133.85, 133.76, 132.95, 132.04, 127.96, 127.46, 120.01 (overlap, two peaks). Anal. calcd for C13H10O2: C, 78.77; H, 5.09. Found: C, 78.37; H, 4.96.
1) as eluent to afford the highly pure product (Table 2). 2-Hydroxybenzophenone9 (Table 2, entry 1) [117–99–7]: IR (KBr): 3500, 1728 cm−1. 1H-NMR (250 MHz, CDCl3) δ (ppm): 11.96 (s, 1H, OH), 7.62–7.40 (m, 7H, Ar), 7.02–6.98 (m, 1H, Ar), 6.83–6.77 (m, 2H, Ar). 13C-NMR (62.9 MHz, CDCl3) δ (ppm): 200.01, 158.02, 140.77, 133.85, 133.76, 132.95, 132.04, 127.96, 127.46, 120.01 (overlap, two peaks). Anal. calcd for C13H10O2: C, 78.77; H, 5.09. Found: C, 78.37; H, 4.96.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18890c |
| This journal is © The Royal Society of Chemistry 2015 |



