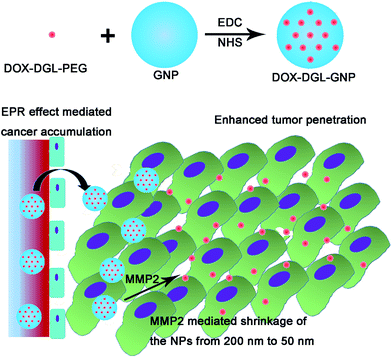
Multistage drug delivery system based on microenvironment-responsive dendrimer–gelatin nanoparticles for deep tumor penetration†
Guanlian Hua,
Yang Wanga,
Qin He*a and
Huile Gao*ab
aKey Laboratory of Drug Targeting and Drug Delivery Systems, West China School of Pharmacy, Sichuan University, No. 17, Block 3, Southern Renmin Road, Chengdu 610041, China. E-mail: gaohuile@scu.edu.cn; qinhe@scu.edu.cn
bState Key Laboratory of Molecular Engineering of Polymers (Fudan University), Shanghai, 200433, China
First published on 28th September 2015
Abstract
A multistage drug delivery system was designed, which showed MMP-2 sensitive shrinkage and enhanced penetration properties.
Cancer is the second leading cause of death in the world and the high recurrence and mortality rates make it one of the greatest challenges in healthcare.1–3 A successful nanoparticle (NP)-based strategy for the treatment of cancer is to not only effectively deliver drugs to cancer in order to improve the antitumor effect but also reduce drug-originating side effects.4 A variety of NPs are commercially available, such as Doxil and Abraxane.5 They accumulate in tumor tissues mainly relying on the enhanced permeability and retention (EPR) effect.6,7 However, because of the dense tumor extracellular matrix (ECM), abnormalities and shortages in the tumor vasculature and high interstitial fluid pressure (IFP), these NPs of fixed size just passively accumulated around the leaky tumor vasculature and could not be delivered throughout the entire tumor tissue with a homogeneous drug concentration.8,9 All these microenvironments of the tumor mean the drugs cannot be effectively delivered to all cancer cells throughout the tumor.10,11 Therefore, constructing a drug delivery system which can not only be retained in the tumor but also possess good penetration efficiency to the deep tumor tissue is of great importance to cancer treatment.
Particle size has a great effect on the tumor penetration and retention of NPs.12–16 Generally, the smaller the particle size is, the stronger the tumor penetration ability the NPs possess.17,18 In contrast, the tumor retention of NPs was positively related with particle size, which meant NPs with larger size (100–200 nm) had a significantly better tumor retention than smaller NPs.12,19,20 The requirements for tumor penetration and tumor retention with regard to particle size were contradictory to each other, and the conventional drug delivery systems could not satisfy them both successfully.
To reconcile this contradiction, multistage drug delivery systems were proposed with a shrinkable size in response to specific stimuli in the tumor,8,21,22 including low pH22–24 and high concentrations of matrix metalloproteinases (MMPs),8 or external stimuli such as irradiation.11,20 Dendritic poly-L-lysine (DGL) is a kind of dendrimer with a size as small as 5 nm.25 Due to the small size of DGL, it may have strong penetrability into tumor tissues. Thus DGL was utilized as a drug carrier in this study. Meanwhile, gelatin is an animal-source compound that could be effectively hydrolyzed into small biomolecules by gelatinases, including MMP-2 and MMP-9,26,27 which are highly expressed by almost all tumors and which have been widely used as stimuli to trigger responsive NPs,28,29 thus in this study gelatin NPs (GNPs) were used as degradable cores. Therefore, doxorubicin (DOX, used as a model drug) decorated DGL was conjugated on the surface of GNPs to construct a MMP-2 sensitive shrinkable system: DOX–DGL–GNP (Fig. 1). The system, with its relatively large size, benefited from the EPR effect, and could effectively target and be retained in a tumor. When DOX–DGL–GNPs reached the dense interstitial matrix by extravasating from the leaky vessels in the tumor after a long circulation, MMP-2 hydrolyzed the gelatin core into small molecules and thereby released actively targeted dendrimers with a small size, significantly increasing their diffusional ability in the interstitial matrix and penetration into the core of the tumor tissue.
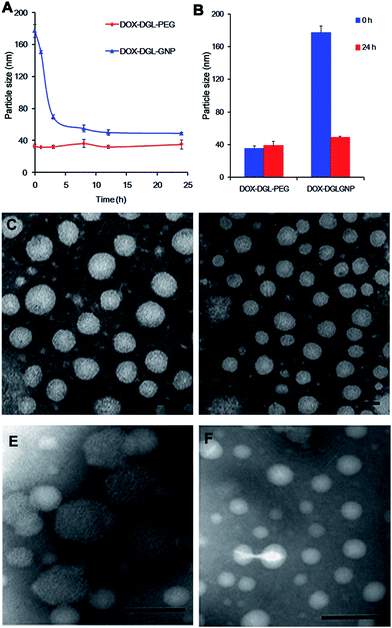
The diameter of DOX–DGL–PEG was approximately 30 nm (Table S1†). After it was decorated onto the GNPs, the diameter of the DOX–DGL–GNPs was considerably increased to approximately 180 nm with a relatively narrow particle distribution. The increase in particle size indicated that the small-sized DOX–DGL–PEG was successfully conjugated on the surface of the GNPs, which was consistent with our previous study.30
In order to verify whether MMP-2 could degrade the GNPs or not, the particle sizes of the DOX–DGL–GNPs and DOX–DGL–PEG were recorded during incubation with MMP-2 (460 ng mL−1) at 37 °C. Before incubation with MMP-2, the size of the DOX–DGL–GNPs was 177.0 ± 5.4 nm. During an MMP-2 incubation ranging from 0 h to 24 h, the particle size was gradually decreased to 48.9 ± 1.4 nm (Fig. 2A, B, E and F), suggesting the incubation with MMP-2 could effective make the DOX–DGL–GNPs shrink from a large size to a small size. In contrast, the size of DOX–DGL–PEG was stable (about 30 nm) during the incubation with MMP-2 (Fig. 2A–D), indicating that the MMP-2 could not degrade the DGL. The result suggested the multistage nanocarrier DOX–DGL–GNPs possessed MMP-2 sensitive shrinkable properties.
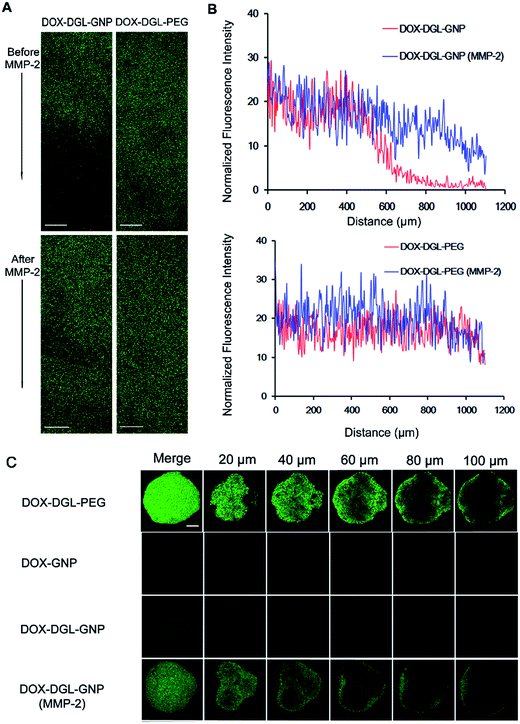
Multicellular tumor spheroids (MCTs) were used to investigate the penetration efficiency of this multistage DOX–DGL–GNP triggered by MMP-2 in a vivo-like tumor. DOX is a small compound that should have a good penetration ability, but in vivo, most of the free drug would be directly trapped in the cells near the vasculature, thus the free DOX could not penetrate into the area far from vasculature.31 However, in vitro, the concentration is much higher than that in vivo, and the drug directly contacts the MCT, which would produce a better penetration effect than NPs.32 Since the in vitro penetration of free drug could not reflect the in vivo condition, free DOX was not used as a control in this study. The released DOX–DGL–PEG conjugates from DOX–DGL–GNPs were able to penetrate as deep as small-sized DOX–DGL–PEG conjugates (Fig. 3C). DOX–DGL–PEG showed higher intensity in all slices of the MCTs while the intensity of DOX–GNP treated MCTs was much lower, suggesting smaller-sized particles possessed better penetrating efficiency.5 By comparison, the fluorescence of DOX–DGL–GNPs incubated with MMP-2 was located inside the MCTs while the fluorescence of DOX–DGL–GNPs was limited at the periphery of the MCTs, this phenomenon was significantly obvious at 100 μm from the surface towards the core. The results confirmed that this multistage nanocarrier DOX–DGL–GNP could benefit from degradation by MMP-2, which facilitated penetration in a tumor by virtue of its then small size.
The small-sized DOX–DGL–PEG could be released from the large-sized DOX–DGL–GNP when degraded by MMP-2, thus significantly decreasing its diffusional hindrance in the tumor dense matrix, and that is the reason why this multistage nanocarrier with a shrinkable size could deliver drug to the tumor’s poorly accessible regions. To verify this hypothesis, a collagen gel was used to simulate the dense interstitial matrix of tumor tissue in vitro.8,21,23 A confocal microscope was used to gain insight into the infiltration activities of each sample into the collagen gel. The collagen gel penetration of large-sized DOX–DGL–GNPs before or after incubation with MMP-2 was compared with small-sized DOX–DGL–PEG as a control. DOX–DGL–GNPs exhibited considerably negligible penetration before degradation (Fig. 3A). In contrast, after being degraded by MMP-2 the DOX–DGL–GNPs were able to penetrate to about a 500 μm depth into the gel (Fig. 3B). Besides, the released DOX–DGL–PEG exhibited similar infiltration activities as the free DOX–DGL–PEG (Fig. 3B). The results demonstrated the penetration ability of this multistage DOX–DGL–GNP in a collagen matrix significantly increased after cleavage by MMP-2, which was consistent with the MCT penetration study.
There are many studies which have been published that reported stimuli responsive NPs, including those which produced drug release, a size change, morphology alternation, etc.28,29 Among which, shrinkable NPs have gained much attention because of their superiority in improving tumor penetration and retention. Previously, we had demonstrated that gold NPs combined with GNPs could enhance the tumor targeting effect of gold NPs.30,33 In this study, we further demonstrated that polymeric NPs and DGL could also be effectively combined with GNPs, resulting in a shrinkable system, which could be used as a good platform for drug delivery. However, this was only a preliminary study, further evaluation, including the in vitro and in vivo distribution, toxicity and antitumor effects should be performed to fully evaluate the constructed NPs.
In summary, a multistage nanocarrier, DOX–DGL–GNP, was constructed and evaluated. Based on the MMP-2 sensitive shrinking, the DOX–DGL–GNPs delivered DOX to the least accessible area of a solid tumor, the core of the tumor tissues. We believe that this study provides a facile strategy towards the design of more intelligent nanocarriers for deep tumor penetration in the future.
Materials and methods
Synthesis of DOX–DGL–PEG conjugates
Detailed information about the synthesis of DOX–DGL–PEG can be found in ESI.† Firstly, cis-aconityl doxorubicin (CAD) was synthesized by using mature reaction steps.34 Secondly, DGL was reacted with NHS–PEG3400 at the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (molar ratio) in PBS (pH 8.0) for 2 h, next the mixture was purified by ultrafiltration.35–37 Next DGL–PEG was freeze-dried and analyzed in a 400 MHz spectrometer. Finally, the carboxyl groups of CAD (42.0 mg, 60 μmol) were reacted with the amino groups of PEG–DGL in the presence of EDC (28.2 mg, 209 μmol) and NHS (12.4 mg, 106 μmol) in the dark for 4 hours,38 after addition of PEG–DGL in 3 mL of PBS (CAD
8 (molar ratio) in PBS (pH 8.0) for 2 h, next the mixture was purified by ultrafiltration.35–37 Next DGL–PEG was freeze-dried and analyzed in a 400 MHz spectrometer. Finally, the carboxyl groups of CAD (42.0 mg, 60 μmol) were reacted with the amino groups of PEG–DGL in the presence of EDC (28.2 mg, 209 μmol) and NHS (12.4 mg, 106 μmol) in the dark for 4 hours,38 after addition of PEG–DGL in 3 mL of PBS (CAD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG–DGL = 48
PEG–DGL = 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, molar ratio), the mixture was kept reacting in the dark for 12 h. Then the mixtures were purified by ultrafiltration through a membrane (MWCO 10
1, molar ratio), the mixture was kept reacting in the dark for 12 h. Then the mixtures were purified by ultrafiltration through a membrane (MWCO 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), and the amount of DOX conjugation was confirmed by UV-Vis spectrophotometry at 480 nm.34
000), and the amount of DOX conjugation was confirmed by UV-Vis spectrophotometry at 480 nm.34
Preparation of GNPs
GNPs were prepared by a two-step desolvation method as previously described with some modification.8 12.5 mL of acetone was added to the gelatin type A (5% w/v, 12.5 mL) solution at 6.0 mL min−1. Exactly 1 min after finishing the addition, the white colored supernatant was discarded and the gel-like precipitate was redissolved in deionized water (8 mL) at 40 °C. Next the pH value was adjusted to 2.7 with HCl (1 M).27 Under constant stirring at 600 rpm and 40 °C, acetone was slowly added at 1 mL min−1 until the solution appeared white milk-like, then glutaraldehyde solution (25%, 60 μL) in acetone (1 mL) was added at 0.05 mL min−1. Subsequently, the solution was kept at 40 °C and 600 rpm for 7 h. At last, the acetone was removed by a rotary evaporator and the remaining solution was filtered through a 0.22 μm syringe filter. Glycine solution (1 M, 0.2 mL) was added and the GNPs were stored overnight at 4 °C until used. A 1 mL solution of the GNPs was injected into a Sephadex G-50 column (3.0 × 50 cm) eluted with PBS. The concentration of solid material in the suspension was usually as high as 20–25 mg mL−1.Preparation of DOX–DGL–GNPs
EDC (0.8 mg, 4.2 μmol) and NHS (0.8 mg, 3.8 μmol) were added to the GNP solution (pH 6.0, 1 mL).8 After 30 min the pH value was adjusted to 8.0, and a solution of COOH–PEG5000–NH2 (20 mg, ≈4 μmol) was added to the mixture. Next the reaction was carried out for 2 h and the pH was adjusted to 6.0, then an additional solution of EDC (0.8 mg) and sulfo-NHS (0.8 mg) dissolved in 50 μL of DI water was added. After stirring for 30 min, the pH value was adjusted to 8.0 again,8 and DOX–DGL–PEG (equivalent to 0.5 mg DOX, 1 mL) was added to the resulting mixture. The mixtures were purified by ultrafiltration through a membrane (MWCO 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) (4500g × 30 min).
000) (4500g × 30 min).
Degradation of GNPs triggered by MMP-2 in vitro
The size change of DOX–DGL–PEG or DOX–DGL–GNPs in response to MMP-2 was measured by a dynamic light scattering detector (Nano-ZS, Malvern, UK). DOX–DGL–PEG or DOX–DGL–GNPs (0.2 mg) were incubated with 460 ng MMP-2 (50 mM Hepes, 2 mM CaCl2) at 37 °C with gentle shaking for 24 h. The morphology of the DOX–DGL–PEG or DOX–DGL–GNPs was described via TEM.Penetration assay using MCTs
The in vitro 4T1 MCTs were assembled using the liquid overlay method. A certain amount of low melting point agarose was dissolved in RPMI 1640 (2%, m/v) and the solution was heated at 80 °C until completely dissolved. 100 μL of the sterile agarose solution (2%, m/v) was added into each well of 96-well plates. Subsequently, 4T1 cells were seeded into each well at the density of 8 × 103 cells per well. The 96-well plates were gently shaken after seeding. The MCTs were allowed to grow up to a diameter of about 300–400 μm for 2 days at 37 °C and 5% CO2 in an incubator. The uniform and compact MCTs were selected for the following studies. The MCTs were treated with different formulations at a DOX concentration of 12.5 μg mL−1 for 24 h. Then, the MCTs were carefully washed thrice with cold PBS (pH 7.4), fixed with 4% paraformaldehyde for 30 min and placed in 96-well plates for confocal observation. The semi-quantitative analysis of the mean fluorescence intensity of DOX in the MCTs was obtained by the confocal laser microscopy software.Collagen gel diffusion
To further explore whether the tumor penetration of the multistage nanocarrier increased or not after degradation, collagen hydrogels were used to simulate the dense matrix of the tumor tissue, which were prepared by mixing 141.75 μL rat tail collagen I (about 4 mg mL−1), 3.8 μL sodium hydroxide (1 M) and 19.5 μL EDTA (0.17 M) on ice.8 After vortexing for 5 min, the collagen hydrogels were added to partially fill a microslide capillary tube followed by a 12 h incubation at 37 °C. DOX–DGL–PEG or DOX–DGL–GNPs (0.1 mg) were incubated with 230 ng of activated MMP-2 in 50 mM Hepes and 2 mM CaCl2 for 24 h. At the end of 24 h, EDTA was added to adjust to a final concentration to 20 mM. 20 μL of the samples was carefully added into the capillary tubes and the whole process of adding samples ensured each sample was fully in contact with the surface of the gel. The sample was left at 37 °C for 12 h and then used to observe the distribution of the fluorescence intensity of DOX via confocal laser scanning microscopy at a wavelength of 560–590 nm.Acknowledgements
This work was supported by the National Natural Science Foundation of China (81402866, 81202926) and the National Basic Research Program of China (973 Program, 2013CB932504).References
- D. A. Corley, C. D. Jensen, A. R. Marks, W. K. Zhao, J. K. Lee, C. A. Doubeni, A. G. Zauber, J. de Boer, B. H. Fireman, J. E. Schottinger, V. P. Quinn, N. R. Ghai, T. R. Levin and C. P. Quesenberry, N. Engl. J. Med., 2014, 370, 1298–1306 CrossRef CAS PubMed.
- H. Z. Chen, S. Y. Tsai and G. Leone, Nat. Rev. Cancer, 2009, 9, 785–797 CrossRef CAS PubMed.
- W. Lu, S. R. Arumugam, D. Senapati, A. K. Singh, T. Arbneshi, S. A. Khan, H. Yu and P. C. Ray, ACS Nano, 2010, 4, 10 Search PubMed.
- J. N. Benjamin le Droumaguet, D. Brambilla, S. Mura, A. Maksimenko, E. S. Line de Kimpe, C. Zona, C. Airoldi, M. Canovi, M. Gobbi, B. L. F. Magali Noiray, F. Nicotra, W. Scheper, O. Flores, M. Masserini and a. P. C. Karine Andrieux, ACS Nano, 2012, 6, 5866–5879 CrossRef PubMed.
- H. Cabral, Y. Matsumoto, K. Mizuno, Q. Chen, M. Murakami, M. Kimura, Y. Terada, M. R. Kano, K. Miyazono, M. Uesaka, N. Nishiyama and K. Kataoka, Nat. Nanotechnol., 2011, 6, 815–823 CrossRef CAS PubMed.
- X. Guo, C. Shi, J. Wang, S. Di and S. Zhou, Biomaterials, 2013, 34, 4544–4554 CrossRef CAS PubMed.
- X. Wang, X. Zhen, J. Wang, J. Zhang, W. Wu and X. Jiang, Biomaterials, 2013, 34, 4667–4679 CrossRef CAS PubMed.
- C. Wonga, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2427 Search PubMed.
- S. Park, S. Kang, X. Chen, E. J. Kim, J. Kim, N. Kim, J. Kim and M. M. Jin, Biomaterials, 2013, 34, 598–605 CrossRef CAS PubMed.
- A. I. Minchinton and I. F. Tannock, Nat. Rev. Cancer, 2006, 6, 583–592 CrossRef CAS PubMed.
- R. Tong, H. H. Chiang and D. S. Kohane, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 19048–19053 CrossRef CAS PubMed.
- C. He, Y. Hu, L. Yin, C. Tang and C. Yin, Biomaterials, 2010, 31, 3657–3666 CrossRef CAS PubMed.
- B. Devika Chithrani, A. A. Ghazani and W. C. W. Chan, Nano Lett., 2006, 6, 662–668 CrossRef PubMed.
- D. Ding, J. Wang, Z. Zhu, R. Li, W. Wu, B. Liu and X. Jiang, ACS Appl. Mater. Interfaces, 2012, 4, 1838–1846 CAS.
- E. Vlashi, ACS Nano, 2013, 7, 8573–8582 CrossRef CAS PubMed.
- L. Tang, N. P. Gabrielson, F. M. Uckun, T. M. Fan and J. Cheng, Mol. Pharm., 2013, 10, 883–892 CrossRef CAS PubMed.
- D. He, J. Y. Shu, J. W. Seo, L. M. Mahakian, K. W. Ferrara and T. Xu, ACS Nano, 2012, 6, 5320–5329 CrossRef PubMed.
- S. Huo, H. Ma, K. Huang, J. Liu, T. Wei, S. Jin, J. Zhang, S. He and X. J. Liang, Cancer Res., 2013, 73, 319–330 CrossRef CAS PubMed.
- S. D. Perrault, Nano Lett., 2009, 9, 1909–1915 CrossRef CAS PubMed.
- R. Tong, H. D. Hemmati, R. Langer and D. S. Kohane, J. Am. Chem. Soc., 2012, 134, 8848–8855 CrossRef CAS PubMed.
- J. Li, Y. Han, Q. Chen, H. Shi, S. ur Rehman, M. Siddiq, Z. Ge and S. Liu, J. Mater. Chem. B, 2014, 2, 1813 RSC.
- Y. Yu, X. Zhang and L. Qiu, Biomaterials, 2014, 35, 3467–3479 CrossRef CAS PubMed.
- M. Zan, J. Li, S. Luo and Z. Ge, Chem. Commun., 2014, 50, 7824–7827 RSC.
- Z. Cheng, Y. Dai, X. Kang, C. Li, S. Huang, H. Lian, Z. Hou, P. Ma and J. Lin, Biomaterials, 2014, 35, 6359–6368 CrossRef CAS PubMed.
- N. Yevlampieva, A. Dobrodumov, O. Nazarova, O. Okatova and H. Cottet, Polymers, 2012, 4, 20–31 CrossRef CAS PubMed.
- J. H. Xu, F. P. Gao, X. F. Liu, Q. Zeng, S. S. Guo, Z. Y. Tang, X. Z. Zhao and H. Wang, Chem. Commun., 2013, 49, 4462–4464 RSC.
- T. G. Shutava, S. S. Balkundi, D. P. O’Neal, Y. M. Lvov, P. Vangala, J. Steffan and R. L. Bigelow, ACS Nano, 2009, 3, 1877–1885 CrossRef CAS PubMed.
- M. R. Dzamukova, E. A. Naumenko, Y. M. Lvov and R. F. Fakhrullin, Sci. Rep., 2015, 5, 10560 CrossRef CAS PubMed.
- A. P. Blum, J. K. Kammeyer, A. M. Rush, C. E. Callmann, M. E. Hahn and N. C. Gianneschi, J. Am. Chem. Soc., 2015, 137, 2140–2154 CrossRef CAS PubMed.
- S. Ruan, X. Cao, X. Cun, G. Hu, Y. Zhou, Y. Zhang, L. Lu, Q. He and H. Gao, Biomaterials, 2015, 60, 100–110 CrossRef CAS PubMed.
- A. J. Primeau, A. Rendon, D. Hedley, L. Lilge and I. F. Tannock, Clin. Cancer Res., 2005, 11, 8782–8788 CrossRef CAS PubMed.
- T. Zong, L. Mei, H. Gao, W. Cai, P. Zhu, K. Shi, J. Chen, Y. Wang, F. Gao and Q. He, Mol. Pharm., 2014, 11, 2346–2357 CrossRef CAS PubMed.
- S. Ruan, Q. He and H. Gao, Nanoscale, 2015, 7, 9487–9496 RSC.
- C. Du, D. Deng, L. Shan, S. Wan, J. Cao, J. Tian, S. Achilefu and Y. Gu, Biomaterials, 2013, 34, 3087–3097 CrossRef CAS PubMed.
- Y. Liu, J. Li, K. Shao, R. Huang, L. Ye, J. Lou and C. Jiang, Biomaterials, 2010, 31, 5246–5257 CrossRef CAS PubMed.
- S. An, Y. Kuang, T. Shen, J. Li, H. Ma, Y. Guo, X. He and C. Jiang, Biomaterials, 2013, 34, 8949–8959 CrossRef CAS PubMed.
- S. Huang, K. Shao, Y. Kuang, Y. Liu, J. Li, S. An, Y. Guo, H. Ma, X. He and C. Jiang, Biomaterials, 2013, 34, 5294–5302 CrossRef CAS PubMed.
- S. Zhu, M. Hong, G. Tang, L. Qian, J. Lin, Y. Jiang and Y. Pei, Biomaterials, 2010, 31, 1360–1371 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18833d |
| This journal is © The Royal Society of Chemistry 2015 |