Stability, antioxidant activity and in vitro bile acid-binding of green, black and dark tea polyphenols during simulated in vitro gastrointestinal digestion†
Zhengmei Wu,
Jianwen Teng,
Li Huang,
Ning Xia and
Baoyao Wei*
College of Light Industry and Food Engineering, Guangxi University, Nanning 530004, PR China. E-mail: weibaoy@126.com
First published on 21st October 2015
Abstract
The stability and antioxidant activity of phenolic compounds, as well as the bile acid-binding activity of green, black, raw liubao and aged liubao tea during in vitro gastrointestinal digestion were evaluated. After in vitro gastrointestinal digestion, the total phenolic content of green tea remarkably decreased. However, it increased in the fermented black, raw liubao and aged liubao tea. Meanwhile, the total catechin recovery of green tea was 52.79%, but those of fermented black, raw liubao and aged liubao tea were 100.27%, 92.73%, and 106.02%, respectively. After gastrointestinal digestion, the ABTS cation radical scavenging capacity of black tea and aged liubao tea increased, and the post-fermented liubao tea increased more significantly. The in vitro bile acid-binding by tea extracts of green, black, raw liubao and aged liubao tea was 17.92–43.55%. Therefore, teas have potential for hyperlipidemic prevention associated with cardiovascular disease.
Introduction
Recently, tea products have observed an increasing interest in human life. The teas can be classified as unfermented tea (green tea), semi-fermented (oolong tea), fermented (black tea), and post-fermented (pu-erh tea, liubao tea) on the basis of the production method. In the past decades, people have found that tea products are effective for the prevention of various illnesses.1–3 It has been reported that these effects are attributed to the polyphenol compounds in tea.4 In general, the teas are digested in the gastrointestinal tract. The polyphenol compounds are released during the digestion and are absorbed in the intestinal tract to achieve this specific effect.5 Tenore et al. found that the polyphenol compounds can be decreased by an average of 44.4% during digestion, as well as 91.8% of native catechin in tea.6Bile acid biosynthesis in the intestinal tract plays an important role in maintaining cholesterol homeostasis. Potential cholesterol-lowering and cancer prevention ability of food fractions could be predicted by evaluating the ability of binding bile acid in vitro. Cholestyramine is one kind of anticholesteremic agent, and is usually employed as the positive bile acids binding control in the previous studies.7–9 Both in vitro and in vivo researches indicate that there is a positive correlation between the bile acid binding ability and cholestyramine dosage.10,11 Binding of bile acid and enhancing the bile acid content in faeces have been assumed to be the possibility cholesterol-lowering mechanism of food fractions.7–9 Gong et al. found that the content of cholesterol and bile acid in the faeces of hyperlipidemia mice could be increased by 4.08–2.11 times by the adding of pu-erh tea theabrownin. It indicated that the theabrownin can improve the transformation and discharge of the cholesterol in food.12 Recent researches demonstrated that phenolic substances in tea have the ability of binding bile acid. Ngamukote et al. indicated that the content of bile acid could be reduced by the binding of gallic acid, catechin and epicatechin with taurocholic acid, glycodeoxycholic acid hydrate and turo-ursodesoxycholic acid, which thus reduced the solubility of cholesterol.13 These studies indicate that polyphenols such as catechin, epicatechin, theaflavin and theabrownin are the main constituents affecting the concentration of the bile acid. Thus it can be seen that the polyphenols compounds have the potential to bind with the bile acid, which will consequently inhibit the absorption of cholesterol and increase the excretion of cholesterol and bile acid.
In our previous studies,14,15 we found that the extracts of liubao tea have the effect of reducing lipid and anticoagulation of the hyperlipidemic mice, and the major antihyperlipidemia individual catechin were finally identified. Our present study focused on the binding effect between the major active ingredients in tea (phenolic compounds) and the bile acids. Changes of the phenolic compounds and antioxidant activity were investigated before and after in vitro digestion. The correlations between the individual catechin and the bile acid binding ability were determined. All our studies showed the potential antihyperlipidemia ability of teas.
Experimental
Chemicals
All reagents and chemicals used were either HPLC grade or analytical grade. The water was prepared using a compact ultrapure water system before use. Standards were: GA: (+)-gallic acid was purchased from Sigma-Aldrich (St. Louis, MO, USA). GC: (−)-gallocatechin; EC: (−)-epicatechin; EGCG: (−)-epigallocatechingallate; GCG: (−)-gallocatechin gallate; ECG: (−)-epicatechingallate were obtained from Institute of Beijing YingzeNaxin chemical technology (Beijing, China). Acetonitrile (HPLC grade) was bought from Merck Specialities Private Limited (Indian). Chemicals and reagents used to simulate the gastrointestinal digestion, and bile acid analysis, were: pepsin, pancreatin, glycocholic acid, glycochenocholic acid, glycodeoxycholic acid, taurochenocholic acid, taurodeoxycholic acid and cholestyramine, were purchased from Sigma-Aldrich (St. Louis, MO, USA). The bile acids analysis commercial kits TBA testing kit, Shanghai Juchuang Biotech. Co., Ltd., (Shanghai, China). Folin–Ciocalteu's phenol reagent was bought from Coolaber science & technology (Beijing, China). L-Ascorbic acid (VC) was bought from Tianjing Bodi chemical industry CO., Ltd (Tianjing, China). 2,2-Azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) was bought from Sigma–Aldrich (St. Louis, MO). 1,1-Diphenyl-2-picrylhydrazyl (DPPH) was bought from Tokyo Chemical Industry (St. Portland, Japan).Preparation of extracts
Green tea and black tea samples were obtained from local markets in Nanning City of China as individual tea bags, raw liubao tea (slightly fermented) and 3 years aged liubao (post-fermented) tea were obtained from China Tea CO., Ltd (Wuzhou, China). All teas were ground to powder. A total of 5.0000g powdered sample was mixed with boiled water (50 mL) and incubated at 80–85 °C for 5 min. After cooling, each sample extract was centrifuged, and supernatant was removed into a volumetric flask (50 mL). An additional of deionized water was used to rinse out the centrifuge tube, which was vortexed and centrifuged as before. Supernatant was combined with the previous supernatant and dilute with deionized water to 50 mL. The solution were frozen at −20 °C for further analysis.Content of total polyphenols and catechins in tea
The total phenolic content was determined according to the International Standards Organisation (ISO) ISO 14502-1-2005E, by the Folin–Ciocalteu reagent. Briefly, 1 mL of sample extract was pipetted into a volumetric flask (100 mL) and mixed with distilled water to the mark. 1 mL of the diluted sample was mixed with 5 mL of 10% Folin–Ciocalteu's phenol reagent. After 3–8 min, 4 mL of 7.5% sodium carbonate solution was added to the reaction mixture, then stand for 1 h before spectrometric analysis. The standard curves of various concentrations of gallic acid were used for quantification, and the results were expressed as mg of gallic acid per mL of sample extracts.Analysis of individual catechins content by HPLC, and was performed according to the ISO 14502-2-2005E procedure. 1 mL of the sample extract was diluted to 10 mL with stabilizing solution (10% v/v acetonitrile with 500 μg mL−1 EDTA and ascorbic acid). Polyphenols were purified from the extracts after digestion by solid phase extraction (SPE) using ProElut C18 cartridges (Dikma Technologies, China). The purified solution was filtered through a 0.45 μm nylon membrane filter and put into vials. An aliquot of 10 μL of the solution was injected into the HPLC system by an auto-sampler. The system, comprised of a Waters e2695 Separations Module, a Waters 2998 Photodiode Array Detector, with photodiode array detection at 278 nm, using a ZORBAX Eclipse Plus C18 column (250 mm × 4.6 mm i.d., 5 μm) (Agilent Technologies, USA). Column temperature was set at 35 °C. Mobile phase A, 9% (volume fraction) acetonitrile, 2% (volume fraction) acetic acid with 20 μg mL−1 EDTA. Mobile phase B, 80% (volume fraction) acetonitrile, 2% (volume fraction) acetic acid with 20 μg mL−1 EDTA. The flow rate of the mobile phase was 1 mL min−1. The gradient of mobile phase A as follows: 0–10 min, 100%, 10–25 min, from 100%–68% and kept at this composition for 10 min. Then reset to 100% A and allowed to equilibrate 10 min before the next injection. Catechin and gallic acid were identified by comparison of their retention times and spectra to those of the standards. Quantification of catechin and gallic acid was carried out by integrating the peak areas and using calibration curves. Results were expressed as microgram of each phenolic compound per 1 mL of sample.
Binding of bile acid
where Amixture is the bile acid concentration in the positive blank and Asupernatant is the bile acid concentration in the supernatant.
Antioxidant activity
Statistical analysis
All data are presented as mean ± SD for three in vitro incubations that were analyzed in triplicate. The statistical analyses were performed using SPSS 19.0. Differences of P < 0.05 were considered significant. Data were subjected to 1-way ANOVA, means compared using Tukey's test (P < 0.05). Correlations among the antioxidant capacity, the total polyphenolic content and the bile acids-binding capacity were established using regression analysis at a 95% significance level. The correlations was considered significant when P < 0.05.Results and discussion
Changes of phenolic compounds
The phenolic compounds are characteristic constituents in tea, and they are also the main active ingredient in tea. Changes of total phenolic compounds in green, black, raw liubao and aged liubao tea extracts during the simulated in vitro gastrointestinal digestion were shown in Fig. 1. It can be seen that there was great difference of total phenolic compounds content between different kinds of tea samples. The total phenolic compounds content of green tea was higher than the other teas, and it was higher in liubao tea than that in black tea. Wu et al. showed that the liubao tea have the highest tea polyphenol content than the other dark teas in China.18 It indicated that the potential biological activity of liubao tea may be stronger than black tea though it belongs to post-fermented tea. The content of the total phenolic compounds in green tea (unfermented) was reduced by 6.12% after simulated digestion. However, content of the total phenolic compounds in black tea (fermented tea), raw liubao tea (fermented tea) and aged liubao tea (post-fermented) were increased by 10.06%, 3.11% and 26.86%, respectively. This indicated that the total phenolic compounds could be reduced in unfermented teas and be increased in fermented tea after the simulated in vitro gastrointestinal digestion. In addition, content of the total phenolic compounds increased with the increase of the fermentation degree. The decrease of the total phenolic compounds may be because of the degradation of catechins during the in vitro gastric and small intestinal digestion.19,20 However, the phenolic polymers in fermented teas will be degraded during the gastrointestinal digestion, which thus produced more phenolic hydroxyl.21,22Catechin content of tea samples
There was a positive correlation relationship between the catechin levels and tea radical scavenging activity. The high catechin levels may be responsible for the protective effects of tea in conditions related to oxidative stress, neoplastic transformations and cardiovascular disease.23,24 Changes of catechin contents in green, black, raw liubao and aged liubao tea after simulated digestion were shown in Fig. 2 and 3 and ESI 1.† It indicated that total catechin recovery of green, black, raw liubao and aged liubao tea were 52.79%, 100.27%, 92.73% and 106.02%, respectively, after gastric intestinal digestion. It can be seen from Fig. 3 and 4 that the total catechin contents of green, black, raw liubao and aged liubao tea were 5042.82 μg mL−1, 1478.08 μg mL−1, 2253.76 μg mL−1 and 1803.77 μg mL−1, respectively. The content of individual catechin was decreased the most in green tea after gastric intestinal digestion. However, content of individual catechin was more stable in fermented tea and increased with the increasing period of fermentation. After the digestion, individual catechin content of GA, GC, EC, EGCG, GCG, ECG were all decreased in green tea. GA, GC, EC, ECG were decreased and EGCG and GCG were increased in black tea. GA, GC, EGCG were decreased and EC, GCG and ECG were increased in raw liubao tea. Furthermore, GA, GC, EC, EGCG, GCG, ECG were all increased in aged liubao tea. Many studies have proposed that catechin will degrade during the in vitro simulated gastro-intestinal digestion,19,20 these studies have shown that the catechins in both green and black teas will be obviously degraded at alkaline pH. This degradation isn't probably due to the decrease of antioxidant activity or total polyphenolic content, which maybe because of the formation of dimers. Our results suggested that the catechins of fermented tea water extracts will degrade and regenerate in gastrointestinal tract. This maybe attribute to the phenolic polymers that generated during the fermentation process. The phenolic polymers will be degraded further and individual catechins and other compounds will generate.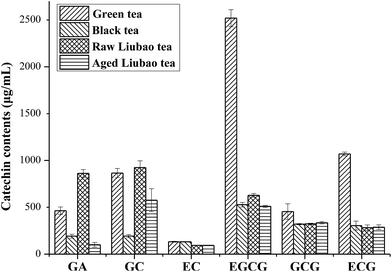 | ||
| Fig. 2 Catechin content of tea samples. Catechin contents are expressed as mean value (μg mL−1 tea extracts) ± SD (n = 3). | ||
 | ||
| Fig. 3 Gastro-intestinal bioaccessibility of catechin from tea samples. Catechin contents are expressed as mean value (μg mL−1 tea extracts) ± SD (n = 3). | ||
Antioxidant activity of tea extracts
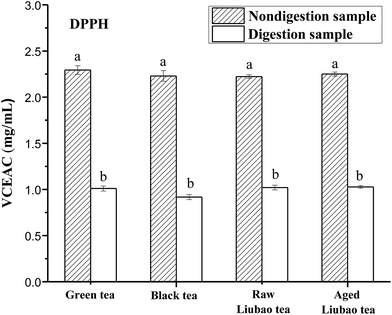
The antioxidant activities of the tea extracts were usually expressed as Vitamin C Equivalent Antioxidant Capacity (VCEAC) in mg mL−1. ABTS and DPPH are two kinds of stable radical species that usually employed for antioxidant activity measurements of the plant extracts.25,26 They are commonly used independently to evaluate their efficacies. Aura et al. indicated that the surviving phenolic compounds during the gastric-intestinal digestion were likely to reach the colon. These phenolic compounds will act as antioxidants or be bio-transformed into phenolic antioxidants and be absorbed in the large intestine/colon.27 Our results as shown in Fig. 4 and 5 indicated that the digestion products still possessed certain antioxidant activities and the order was nondigestion sample > digestion sample, except ABTS cation radical scavenging capacity of black tea and aged liubao tea.DPPH radical scavenging activity of green, black, raw liubao and aged liubao tea showed a similar tendency during the in vitro gastro-intestinal digestion as shown in Fig. 4. The DPPH radical scavenging activity of green, black, raw liubao and aged liubao tea were decreased by 55.9%, 58.7%, 54.0% and 54.2%, respectively. It indicated that the DPPH radical scavenging activity of all tea extracts remarkably decreased after vitro gastro-intestinal digestion. This could be due to the fermenting degrees make no difference to the DPPH scavenging activity of tea, or it may be that the oxidation resultant products also have remarkable effect on the antioxidant activity.28
ABTS cation radical scavenging capacity of green, black, raw liubao and aged liubao tea were shown in Fig. 5. During vitro gastro-intestinal digestion, ABTS cation radical scavenging capacity of green and raw liubao tea were decreased by 10.7% and 36.45%, respectively. However, ABTS cation radical scavenging capacity of black and aged liubao tea were increased by 22.9% and 26.0%, respectively. It could be speculated that the tea polyphenols polymerides structure will be changed and will be degraded during its transportation from intestine. The resultant small molecular phenolic compounds and breakdown products might have a higher ABTS cation radical scavenging capacity, which will directly contribute to the antioxidant capacity of the digestion samples. Mukai et al. indicated that the increase of the radical scavenger activity was attributed to the deprotonation of the hydroxyl moieties present on the aromatic rings of the phenolic compounds.29 The phenolic molecules structure will be changed during the transition from the stomach to the intestinal environment, which is attributed to the ionisation of the hydroxyl groups.30
In vitro bile acid binding by tea extract
Cholestyramine is a bile acid sequestrant. It is positively charged non-digestible resins that bind to bile acids in the intestine to form an insoluble complex.31 In our research, cholestyramine bound 95% of the bile acids. It is higher than the previous studies, Story & Kritchevsky found that 81% bile acid binding by cholestyramine using 50 mg of substrate and 50 μmol of bile acids.32 It may be that the use of the higher substrate to bile acid ratio. In vitro bile acid binding by tea extracts on equal volume of tea extract was shown in Table 1. Green, black, raw liubao and aged liubao tea bound 1.59, 0.46, 0.65 and 0.90 μM of bile acid per milliliter of sample, respectively, which was equal to 43.55, 12.54, 17.92 and 24.73% of the total added BA. Assigning a bile acid binding value of 100% to cholestyramine (50 mg), the relative bile acid binding on 1 mL for the test samples of tea extract was green tea 45.83%, black tea 13.20%, raw liubao tea 18.86%, and aged liubao tea 26.03%. Bile acid binding for green tea was significantly higher than the other three teas. Relative bile acid binding on tea extract was green tea > aged liubao tea > raw liubao tea > black tea. The different bile acid binding may be associate with the phytonutrients (antioxidants, polyphenols, hydroxycinnamic acids, flavonoids, anthocyanins, flavonols, proanthocyanidins, catechins), hyrophobicity or active binding sites of the various tea.9 On a tea extract, bile acid binding of 17.92–43.55% for green, black, raw liubao and aged liubao tea are very encouraging and could indicate health promoting benefits of these teas. Health properties of the food fractions were generally evaluated by testing their bile acid binding. Fat absorption, cancer causing toxic metabolites excretion and cholesterol utilization to synthesize more bile acids will be reduced by bile acids binding. It is supposed to be the lower cholesterol and prevent cancer mechanism by food fractions. Previous studies reported for fresh fruits and fresh green vegetables, blueberries 7%, plums 6%, prunes 5%, strawberries 5%, cherries 5%, cranberries 4%, apples 1%, broccoli 5%, mustard greens 4%, and potato cultivars as raw, steamed or steamed then cooled range from 20.55% to 36.50% under similar conditions.9,33 It showed that bile acid binding capacity by tea extracts is significant higher than a variety of fruits and vegetables.| Treatment | Bile acid binding | ||
|---|---|---|---|
| (μmol mL−1 tea extracts) | Percentage bile acid binding (%) | Relative to cholestyramine (50 mg), % | |
| a Mean ± SEM within a column with different superscript letters differ significantly (P ≤ 0.05), n = 3. | |||
| Green tea | 1.59 ± 0.25a | 43.55 ± 6.84a | 45.83 ± 7.20a |
| Black tea | 0.46 ± 0.02b | 12.54 ± 7.93b | 13.20 ± 8.34b |
| Raw liubao tea | 0.65 ± 0.02b | 17.92 ± 5.30b | 18.86 ± 5.58b |
| Aged liubao tea | 0.90 ± 0.02b | 24.73 ± 4.56b | 26.03 ± 4.80b |
Correlation analysis
Correlation analysis was employed to go to show the relationship among total polyphenolic content, scavenging activities and bile acids-binding capacity. The correlation analysis was measured for all samples during vitro gastro-intestinal digestion and was presented in Table 2. It indicated that the correlation between TP contents and DPPH activity was poor. This may be due to TP did not have the effect of the DPPH scavenging activity of tea.34 It meant that TP contribute less to the DPPH antioxidant capacity of fermented teas. There was a positive correlation relationship between the individual phenolic compounds and the DPPH scavenging activity. This may be due to the structure changing of the phenolic compounds during the in vitro gastrointestinal digestion, and the newly produced compounds also contribute to the DPPH antioxidant capacity.19 However, highly significant positive correlations of TP, GA, GC contents with ABTS cation radical scavenging capacity were observed. This result was similar to the earlier research that stated that TP content had correlation coefficients (r = 0.836) with ABTS.35 The other individual catechins also have positive correlations with ABTS cation radical scavenging capacity, but not significant. Chang et al. indicated that the phenolic contents in plants contributed to antioxidant activity probably because of their redox properties, where the phenolic contents acted as reducing agents, hydrogen donors and singlet oxygen quenchers.36| TP | GA | GC | EC | EGCG | GCG | ECG | DPPH scavenging capacity | ABTS cation radical scavenging capacity | |
|---|---|---|---|---|---|---|---|---|---|
| a Significant at 5% level. | |||||||||
| DPPH scavenging capacity | −0.16 | 0.320 | 0.173 | 0.11 | 0.353 | 0.046 | 0.248 | ||
| ABTS cation radical scavenging capacity | 0.802a | 0.819a | 0.827a | 0.04 | 0.612 | 0.593 | 0.626 | 0.367 | |
| Bile acids-binding capacity | 0.947a | 0.042 | 0.571 | 0.300 | 0.924a | 0.960a | 0.923a | 0.970a | 0.561 |
There was a significant positive correlation between TP, EGCG, GCG, ECG contents and bile acids-binding capacity. Significant positive correlation between DPPH scavenging capacity and bile acids-binding capacity were also observed. The other individual catechins also have positive correlations with bile acids-binding capacity, but not significant. Ikeda et al. found that tea catechins rich in gallocatechin gallate and catechin gallate were better availability to decrease cholesterol absorption than that rich in epigallocatechin gallate and epicatechin gallate.37 It can thus be seen that the esterifiable catechins have higher bile acids-binding capacity than the unesterified catechins. This was in accordance with our previous results.
Conclusions
The tea has a positive function in health promotion. Phenolic polymers could be degraded and produce individual catechins and some other compounds during the in vitro gastrointestinal digestion of fermented tea extracts. These individual catechins have large contribution to ABTS cation radical scavenging capacity, and the interesting unknown compounds deserve further exploration. The tea extracts possess excellent antioxidant capacity and in vitro bile acid binding capacity, where the liubao tea show more advantage than the other fermented tea. Fermented tea like liubao tea has a wider space in the functional research field, which requires more in-depth research in future.Acknowledgements
This Project was graciously sponsored by the Guangxi Natural Science Foundation (2012GXNSFBA053024), and the Guangxi science and technology development program (159832-05). Gratitude is expressed to Dr Shuangxi Nie of Guangxi University, China, who reviewed and checked this paper carefully.Notes and references
- I. Ikeda, Y. Imasato, E. Sasaki, M. Nakayama, H. Nagao, T. Takeo, F. Yayabe and M. Sugano, Biochim. Biophys. Acta, Lipids Lipid Metab., 1992, 1127, 141–146 CrossRef CAS.
- J. H. Weisburger and F.-L. Chung, Food Chem. Toxicol., 2002, 40, 1145–1154 CrossRef CAS PubMed.
- Q. Huang, S. Chen, H. Chen, Y. Wang, Y. Wang, D. Hochstetter and P. Xu, Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association, 2013, vol. 53, pp. 75–83 Search PubMed.
- J. Y. Kohei Jobu, S. Yoshioka, H. Moriyama, S. Murata, H. Masao Ohishi and M. Miyamura, Food Res. Int., 2013, 54, 324–329 CrossRef.
- P. Xiao, H. Huang, J. Chen and X. Li, J. Ethnopharmacol., 2014, 157, 55–61 CrossRef CAS PubMed.
- G. C. Tenore, P. Campiglia, D. Giannetti and E. Novellino, Food Chem., 2015, 169, 320–326 CrossRef CAS PubMed.
- R.-S. Fátima, R.-G. Guillermo, L.-M. Antonio and F.-B. Juan, Food Hydrocolloids, 2015, 43, 311–321 CrossRef.
- Y. Niu, Z. Xie, J. Hao, W. Yao, J. Yue and L. Yu, Food Chem., 2012, 132, 1025–1032 CrossRef CAS.
- T. Kahlon and G. Smith, Food Chem., 2007, 100, 1182–1187 CrossRef CAS.
- K. E. Suckling, G. M. Benson, B. Bond, A. Gee, A. Glen, C. Haynes and B. Jackson, Atherosclerosis, 1991, 89, 183–190 CrossRef CAS PubMed.
- T. Nakamura and Y. Matsuzawa, Jpn. J. Clin. Med., 1994, 52, 3266–3270 CAS.
- J. Gong, C. Peng, T. Chen, B. Gao and H. Zhou, J. Food Sci., 2010, 75, H182–H189 CrossRef CAS PubMed.
- S. Ngamukote, K. Makynen, T. Thilawech and S. Adisakwattana, Molecules, 2011, 16, 5054–5061 CrossRef CAS PubMed.
- L. Huang, J. Peng, N. Xia, J. Teng and B. Wei, Food Sci. Technol., 2013, 8, 038 Search PubMed.
- J. Peng, J. Guangxi Univ., 2012, 17–28 Search PubMed.
- P. Xiao, H. Huang, J. Chen and X. Li, J. Ethnopharmacol., 2014, 157, 55–61 CrossRef CAS PubMed.
- H. Du, J. Wu, H. Li, P.-X. Zhong, Y.-J. Xu, C.-H. Li, K.-X. Ji and L.-S. Wang, Food Chem., 2013, 141, 4260–4268 CrossRef CAS PubMed.
- C. Wu, J. Hunan Agric. Univ., 2012, 22–36 Search PubMed.
- A. P. Neilson, A. S. Hopf, B. R. Cooper, M. A. Pereira, J. A. Bomser and M. G. Ferruzzi, J. Agric. Food Chem., 2007, 55, 8941–8949 CrossRef CAS PubMed.
- I. R. Record and J. M. Lane, Food Chem., 2001, 73, 481–486 CrossRef CAS.
- J. Bouayed, L. Hoffmann and T. Bohn, Food Chem., 2011, 128, 14–21 CrossRef CAS PubMed.
- M. J. Rodriguez-Roque, M. A. Rojas-Grau, P. Elez-Martinez and O. Martin-Belloso, J. Agric. Food Chem., 2013, 61, 1859–1867 CrossRef CAS PubMed.
- G. C. Tenore, P. Stiuso, P. Campiglia and E. Novellino, Food Chem., 2013, 141, 2379–2384 CrossRef CAS PubMed.
- C. J. Dufresne and E. R. Farnworth, J. Nutr. Biochem., 2001, 12, 404–421 CrossRef CAS PubMed.
- L. G. Ranilla, Y.-I. Kwon, E. Apostolidis and K. Shetty, Bioresour. Technol., 2010, 101, 4676–4689 CrossRef CAS PubMed.
- D. Malenčić, Z. Maksimović, M. Popović and J. Miladinović, Bioresour. Technol., 2008, 99, 6688–6691 CrossRef PubMed.
- A.-M. Aura, P. Martin-Lopez, K. A. O'Leary, G. Williamson, K.-M. Oksman-Caldentey, K. Poutanen and C. Santos-Buelga, Eur. J. Nutr., 2005, 44, 133–142 CrossRef CAS PubMed.
- G. A. A. R. Perera, A. M. T. Amarakoon, D. C. K. Illeperuma and P. K. P. Muthukumarana, LWT–Food Sci. Technol., 2015, 63, 745–750 CrossRef CAS.
- K. Mukai, W. Oka, K. Watanabe, Y. Egawa, S.-I. Nagaoka and J. Terao, J. Phys. Chem. A, 1997, 101, 3746–3753 CrossRef CAS.
- D. Tagliazucchi, E. Verzelloni, D. Bertolini and A. Conte, Food Chem., 2010, 120, 599–606 CrossRef CAS.
- F. Scaldaferri, M. Pizzoferrato, F. R. Ponziani, G. Gasbarrini and A. Gasbarrini, Int. Emerg. Med., 2013, 8, 205–210 CrossRef PubMed.
- J. A. Story and D. Kritchevsky, J. Nutr., 1976, 106, 1292–1294 CrossRef CAS PubMed.
- T. Kahlon, M. Chapman and G. Smith, Food Chem., 2007, 100, 1531–1536 CrossRef CAS.
- S. Jayasekera, A. Molan, M. Garg and P. Moughan, Food Chem., 2011, 125, 536–541 CrossRef CAS.
- L. Wang, S. Su, J. Wu, H. Du, S. Li, J. Huo, Y. Zhang and L. Wang, Food Chem., 2014, 160, 357–364 CrossRef CAS PubMed.
- S. Chang, J. Wu, S. Wang, P. Kang, N. Yang and L. Shyur, J. Agric. Food Chem., 2001, 49, 3420–3424 CrossRef CAS PubMed.
- I. Ikeda, M. Kobayashi, T. Hamada, K. Tsuda, H. Goto, K. Imaizumi, A. Nozawa, A. Sugimoto and T. Kakuda, J. Agric. Food Chem., 2003, 51, 7303–7307 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18784b |
| This journal is © The Royal Society of Chemistry 2015 |