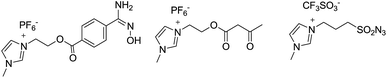
Imidazolium-supported benzotriazole: an efficient and recoverable activating reagent for amide, ester and thioester bond formation in water†‡
S. M. Abdul
Shakoor
,
Sunita
Choudhary
,
Kiran
Bajaj
,
Manoj Kumar
Muthyala
,
Anil
Kumar
and
Rajeev
Sakhuja
*
Department of Chemistry, Birla Institute of Technology and Science, Pilani 333 031, Rajasthan, India. E-mail: sakhuja.rajeev@gmail.com
First published on 21st September 2015
Abstract
An efficient and recyclable imidazolium-supported benzotriazole reagent (Im-CH2-BtH) as a novel synthetic auxiliary has been synthesized and its utility as a carboxyl group activating reagent via the formation of stable imidazolium-supported acyl benzotriazoles was explored for the synthesis of amides, esters and thioesters in water under microwave conditions. The reagent was reused five times without any noticeable loss in activity. It is moisture insensitive and highly stable under thermal and aerobic conditions. The application of imidazolium-supported N-acetyl benzotriazole leads to synthesis of paracetamol on the gram scale under greener conditions in 93% yield.
Introduction
Amide, ester and thioester functionalities are highly desirable synthetic targets that form an integral component of numerous natural products and biologically active synthetic molecules. The amide bond itself is present in more than 25% of known drugs as per the Comprehensive Medicinal Chemistry (CMC) database analysis.1 Amide and ester linkages are introduced as an essential component to synthetic drugs such as procaine, lidocaine, tocainide to increase their metabolic stability.2 These groups impose degradable character and good thermal and mechanical properties to biodegradable poly(ester amide)s (PEAs) polymers.3 Glycolamide esters of aspirin,4 ibuprofen,5 niflumic acid,6 scutellarin7 and nimesulide8 have also been used as biolabile prodrugs due to their ability to undergo quick cleavage in human plasma. Similarly, thioesters have been extensively used in native chemical ligation for the synthesis of many biologically active small- and medium-sized peptides and proteins.9Acid-activation as acyl halides, acyl azides, acylimidazoles, anhydrides, esters and acyl benzotriazoles, followed by nucleophilic substitution are among the most common strategies employed for construction of amide, ester and thioester bonds. N-Acylbenzotriazoles has surpasses most of the aforementioned reagents due to their relative stability, ease of formation, higher reactivity and high yields of the N-, S- and O-acylated products.10 Katritzky's pioneer work on the applications of N-acylbenzotriazoles has led to the synthesis of libraries of novel peptides and peptidomimetics.11 However, the N-acylbenzotriazole methodology suffers one or more hindrances in term of green chemistry perspective such as use of organic solvents in the acylation reaction, non-reusability of benzotriazole, and sometimes purification of the acylated product by column chromatography.12 Efforts to overcome some of these drawbacks have been undertaken by Paio,13a Showalter,13b Fang13c and Katritzky13d,e leading to the synthesis of polymer-supported benzotriazole reagents. Although, polymer-supported benzotriazoles have overcome some of these limitations but they have their own issues such as low loading capacity, elaborate purification procedures, low swelling properties, limited solubility and scope of the reagents.13
In past decade, there has been a great attention on the synthesis of functionalized imidazolium-supported reagents and their use as alternative soluble support in solution-phase parallel synthesis14 and in facilitating the separation process (Fig. 1).15
Tuneable chemical and physical properties, higher loading capacity, reactions under homogeneous conditions and use of conventional methods for the analysis of reaction progress has proved them as ideal support for the reagents16 in the synthesis of small molecule libraries,17 peptides,18 and oligosaccharides.19 With our interest in application of benzotrioazole in acylation reactions and imidazolium-supported reagents we envisioned that supporting benzotriazole on imidazolium support can generate a soluble-supported benzotriazole auxiliary that can have high efficiency and can be easily recycled. In this article, we report synthesis of imidazolium-supported benzotriazole and its application as reusable carboxyl group activating reagent for amide, ester and thioester bond formation in aqueous media under microwave irradiation.
Results and discussion
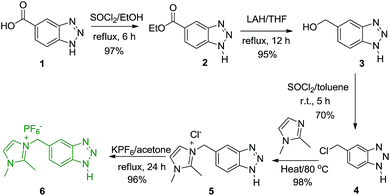
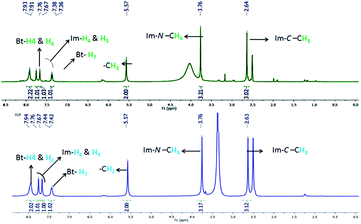
Our work commenced with the synthesis of imidazolium-supported benzotriazole reagent connected via methylene linker (Im-CH2-BtH, 6) as depicted in Scheme 1. Esterification of commercially available benzotriazole-5-carboxylic acid (1), followed by reduction with lithium aluminium hydride (LAH) in THF yielded 5-hydroxymethylbenzotriazole (3).20 Chlorination of 3 with thionyl chloride in toluene furnished 5-chloromethylbenzotriazole (4) in 70% as a single isomer. Reaction of 4 with 1,2-dimethylimidazole under solvent free heating condition afforded imidazolium methylene linked benzotriazole chloride salt (5), as a gummy product in 98% yield. Based on the 1H NMR analysis, it was found that 5 is isomeric mixture of N1- and N3-isomers with major amounts of N1-isomer. It was used as such for the next step without further purification.For tuning the solubility, the counter anion in 5 was exchanged with PF6 anion by reacting with KPF6 in dry acetone under refluxing condition to yield methylene linked imidazolium benzotriazole hexafluorophosphate (6) as a brown solid in 96% yield. Initially the exchange of Cl− by PF6− was attempted in water at room temperature but the reaction never goes on completion and the product remains soluble along with 5 in water. On the other hand, the reaction goes to completion only on refluxing in dry acetone. The 1H NMR of 6 indicated the presence of N1- and N2-isomers in the ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Three characteristic singlet at δ 5.58, 3.76 and 2.64 for the methylene (2H), N-methyl (3H) and C-methyl (3H) protons were observed for N1-isomer along with three singlets at δ 6.17, 3.66 and 2.44 for N2-isomer. Peaks for equivalent aromatic protons were observed in the region δ 7.37–8.07 in the 1H NMR of 6. We were successful in isolating N1-isomer by recrystallization of the brown solid from methanol. Thus, the overall strategy provides a straight forward high yielding method for the synthesis of imidazolium-supported benzotriazole, avoiding protection and de-protection steps as usually required in polymer-supported benzotriazole synthesis.
1. Three characteristic singlet at δ 5.58, 3.76 and 2.64 for the methylene (2H), N-methyl (3H) and C-methyl (3H) protons were observed for N1-isomer along with three singlets at δ 6.17, 3.66 and 2.44 for N2-isomer. Peaks for equivalent aromatic protons were observed in the region δ 7.37–8.07 in the 1H NMR of 6. We were successful in isolating N1-isomer by recrystallization of the brown solid from methanol. Thus, the overall strategy provides a straight forward high yielding method for the synthesis of imidazolium-supported benzotriazole, avoiding protection and de-protection steps as usually required in polymer-supported benzotriazole synthesis.
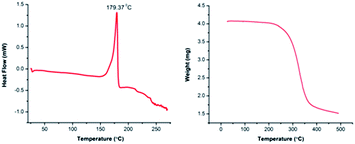
The thermal stability profile of the synthesized reagent 6 was studied using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The DSC experiment showed that the exothermic decomposition temperature of 6 is above 150 °C (Fig. 2, left curve) with an initiation temperature of 151.81 °C and end point at 185.81 °C. We recommend the use of 6 well below its decomposition temperature. It is worth mentioning that 6 has not shown any sign of decomposition or loss of reactivity even after storing for more than two month at room temperature. The TGA of 6 indicated the first weight loss to be around 254 °C and the full degradation centered around 366 °C (Fig. 2, right curve) suggesting that 6 is thermally stable.
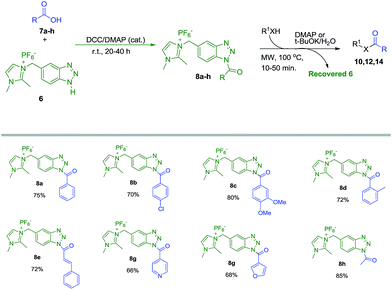
After successful synthesis and characterization of 6, we focused our attention to investigate the carboxylic acid activating capability of 6 to synthesize amides, esters and thioesters. Thus, we initially attempted the activation of benzoic acid (7a) with 6. It is important to mention that initially the activation of 7a was attempted with the reagent 5 (Im-CH2-BtH, Cl− salt), however due to difficulty in the weighing because of gummy nature of the reagent and comparatively lower reactivity, we switched to activation using 6 (Im-CH2-BtH, PF6− salt).
Various coupling reagents viz. DCC, EDC·HCl, HBTU and HATU were screened in tetrahydrofuran (THF) and acetonitrile (CH3CN) for the activation of benzoic acid (7a) with 6 (Table 1). Other polar solvents such as DMF and DMSO were discarded due to difficulty of removing them after the reaction. Among all the trials, DCC (1.2 eq.) in CH3CN using a catalytic amounts of DMAP at room temperature gave best yield (75%) of imidazolium-supported N-benzoyl benzotriazole (8a) (Table 1, entry 3). A number of spots were visible on the TLC when the reaction was carried under refluxing conditions and subsequently leads to declination in the isolated yield of 8a under similar experimental conditions (Table 1, entry 5). Interestingly due to high polarity of 8a, the DCU was easily removed from the reaction mixture by simple washing with ethyl acetate and thereafter precipitation with methanol yielded 8a as pure product. Catalytic amounts of DMAP was enough and necessary for the reaction as its absence leads to tremendous decrease in the yield of 8a (Table 1, entry 4).
| Entry | Coupling reagents | Solvent | Reaction conditions | Yield of 8a (%) |
|---|---|---|---|---|
| a A number of spots on TLC appeared on refluxing and subsequently the yield of 8a decreased. | ||||
| 1 | DCC (1.2 eq.)/DMAP (cat.) | THF | r.t., 26 h | 50 |
| 2 | DCC (2 eq.)/DMAP (cat.) | CH3CN | r.t., 20 h | 76 |
| 3 | DCC (1.2 eq.)/DMAP (cat.) | CH3CN | r.t., 20 h | 75 |
| 4 | DCC (1.2 eq.) | CH3CN | r.t., 20 h | 40 |
| 5 | DCC (1.2 eq.)/DMAP (cat.) | CH3CN | Reflux, 8 h | 55a |
| 5 | EDC·HCl (1.5 eq.)/DMAP (cat.) | CH3CN | r.t., 20 h | 65 |
| 6 | HBTU (1.5 eq.)/DMAP (cat.) | CH3CN | r.t., 20 h | 45 |
| 7 | HATU (1 eq.)/DMAP (cat.) | CH3CN | r.t., 20 h | 52 |
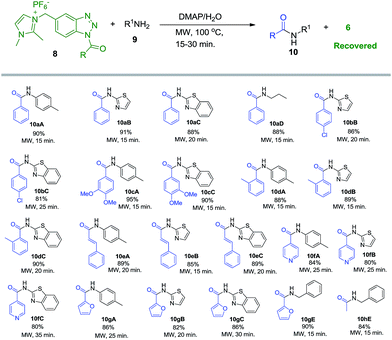
With these optimized conditions in hand, we studied the substrate scope of this reaction and successfully activated six more aromatic and heteroaromatic acids (7b–g) with imidazolium-supported benzotriazole auxiliary yielding their corresponding stable imidazolium-supported benzotriazole acyl reagents (Im-CH2-Bt-COAr, 8b–g) in 65–80% yields (Scheme 2) either in pure N1-isomeric form or enriched N1-isomer with small amounts of N3-isomer. Apart from aromatic and heteroaromatic acids, acetic acid also got efficiently activated by our synthesized reagent 6 yielding corresponding imidazolium-supported benzotriazole acetyl reagent (8h) in 85% isolated yield, under similar experimental conditions in 16 h. It is noteworthy that the loss in yield of 8 is solely during the isolation process due to its partial solubility in methanol.
We next investigated benzoylation of p-toluidine (9A) with imidazolium-supported N-benzoyl benzotriazole (8a) using DMAP and K2CO3 as bases under classical and microwave conditions in a number of solvents such as CH3CN, THF, EtOH and water. To our delight, the reaction in CH3CN and water proceeded smoothly under microwave irradiation giving 10aA in 85% and 90% yields, respectively (Scheme 3). On the other hand, it took 12–14 h for completion of reaction under reflux conditions in acetonitrile and water with almost comparable yields.
 | ||
| Scheme 3 Imidazolium-supported acyl benzotriazole (8a–g) mediated synthesis of amides (10) in aqueous media under MW irradiation. | ||
The substrate scope of other imidazolium-supported N-acyl benzotriazoles (8b–g) was checked under similar conditions with a variety of aliphatic, aromatic and heteroaromatic amines as N-nucleophilic substrates (9A–E). For all substrates, the reaction proceeded with quantitative conversion in water by applying 15–30 minutes of microwave irradiation, and 80–95% isolated yield of the corresponding amides (10) were obtained (Scheme 3). Interestingly, a simple extraction of the concentrated reaction mixture with ethyl acetate followed by washing with 1 N HCl (to remove the un-reacted N-nucleophilic substrate and DMAP) yielded pure amides 10, avoiding any use of column chromatography. Apart from aromatic and heteroaromatic N-nucleophiles, the acylation using 8 worked quite well with aliphatic amines such as propyl amine (9D) and benzyl amine (9E), yielding the corresponding amides 10aD and 10gE in 88% and 90% yields, respectively. Similarly the acetylation of benzyl amine (9E) with 8h proceeded in 15 minutes under similar experimental conditions yielding 10hE in 84% yield.
We further utilized imidazolium-supported N-acyl benzotriazoles (8a–g) for the thioester bond formation by reacting with thiophenol (11A) and 2-naphthalenethiol (11B) under similar conditions (Scheme 4). Here again, DMAP was found to be the optimum base for the reactions in water and acetonitrile and the reaction worked comparatively slow with K2CO3. Longer reaction time and lower yield of the product (12aA) was obtained under classical reflux conditions in water and acetonitrile. Both thiophenols reacted smoothly with 8a–g and the reaction completed in 10–30 minute of microwave irradiation. Extraction of the concentrated reaction mixture with ethyl acetate followed by washing of organic filtrate with water (to remove DMAP) and recrystallization with hexanes yielded pure thioesters (12) in 85–96% yields.
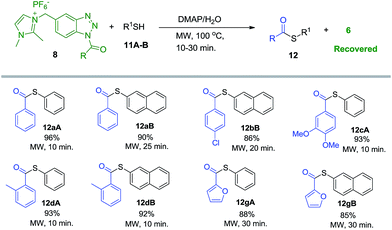 | ||
| Scheme 4 Imidazolium-supported acyl benzotriazole (8a–g) mediated synthesis of thioesters (12) in aqueous media under MW irradiation. | ||
After the success of amide and thioester bond formation in aqueous medium, we then turned our attention towards ester bond formation using imidazolium-supported N-acyl benzotriazoles (8a–g). When 3-methoxyphenol (13) was allowed to react with 8a under the standardized condition for amide bond formation, corresponding ester 14aA was only formed in 26% yield. This may be attributed to the lower nucelophilicity of phenols compare to amines and thiols However, use of t-BuOK instead of DMAP resulted in formation of 14aA in 80% isolated yield after 40 minutes of MW irradiation. Next, when other imidazolium-supported acyl benzotriazole (8b–g) were reacted with 3-methoxyphenol (13) in water under microwave irradiation using t-BuOK as base to give corresponding esters 14aA–gA (Scheme 5). Extraction of the concentrated reaction mixture with ethyl acetate followed by washing of organic filtrate with 2 N NaOH yielded pure esters (14aA–gA) in 73–84% isolated yields.
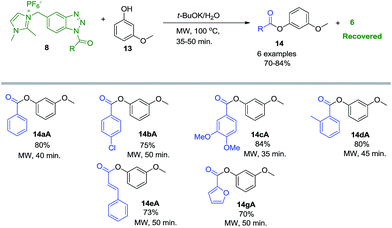 | ||
| Scheme 5 Imidazolium-supported acyl benzotriazole (8a–g) mediated synthesis of esters (14) in aqueous media under MW irradiation. | ||
Recovery and reusability of imidazolium-supported benzotriazole reagent
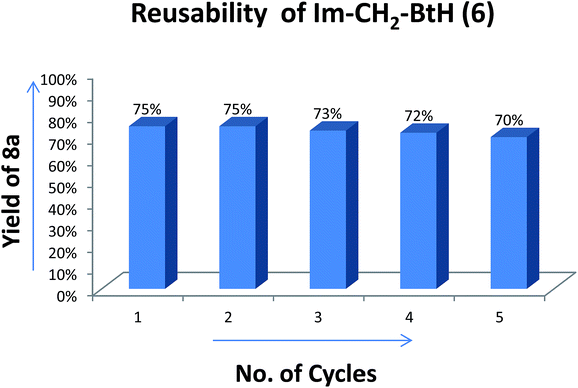
Recoverability of Im-CH2-BtH was studied in all the three cases of reactions. The insolubility of Im-CH2-BtH (6) in ethyl acetate provided a simple procedure for separating it from crude reaction mixture. In case of amide and thioester synthesis, pure Im-CH2-BtH (6) was obtained after washing of concentrated reaction mass with ethyl acetate followed by high vacuum drying whereas in case of ester synthesis an additional washing with water was required to remove t-BuOK to yield pure 6. Comparison of the 1H NMR of the recovered Im-CH2-BtH (6) reagent with that of initially synthesized reagent (6) is shown in Fig. 3. From the 1H NMR it is very clear that the chemical structure of recovered 6 is identical to that of original compound.The reusability of the recovered reagent (6) was evaluated towards the synthesis of 8a. Delightfully the recovered reagent (6) has not shown any significant change in the chemical reactivity and for five cycles 75, 75, 73, 72 and 70% isolated yield of 8a was obtained based on the amount of recovered Im-CH2-BtH (6) (Fig. 4). It is worth to mention that the recovery process is very simple and less arduous. However, due to inevitable loss of Im-CH2-BtH (6) during the activation step, the amount of amide (10aA) formed in each subsequent cycle's decreases (percentage yield remain same) due to use of lesser amount of nucleophile in the subsequent cycles.
One-pot and sequential synthesis of amide without isolating Im-CH2-BtCOR
To overcome the loss in yield during the synthesis of imidazolium-supported acyl benzotriazole reagents (Im-CH2-Bt-COR) (8) and to make the process more efficient and economical, we planned to perform the synthesis of amide (10aA) in one-pot fashion. It is important to mention that since the activation step does not proceed in water, therefore benzoic acid (7a) and Im-CH2-BtH (6) were allowed to react in acetonitrile at room temperature for 20 h using DCC (1.2 eq.)/DMAP (5 mol%), after which p-toluidine (1.0 eq.), additional DMAP (1.2 eq.) were added to the same pot and the reaction mixture was subjected to microwave irradiation for 20 minutes. After the two sequential reactions in one-pot, the reagent Im-CH2-BtH (6) was almost completely recovered (97% of its starting amount) by washing the concentrated and dried reaction mixture with ethyl acetate and decanting the organic filtrate to separate the product along with by-products such as DCU, DMAP and un-reacted acid from 6. However, the isolation of the product 10aA in 82% yield from this organic filtrate (ethyl acetate) required tedious separation procedure including acid–base work up followed by column chromatography.In order to exploit the synthetic advantage of the reagent (Im-CH2-BtCOR) to effectively undergo acylation in water, we also attempted the sequential synthesis of amide (10aA) by (i) carrying the activation step in acetonitrile in the usual manner at room temperature, (ii) removing the side products (DCU) by washing the concentrated and dried residue with ethyl acetate and (iii) using this concentrated residue for the benzoylation of p-toluidine in water using DMAP (1.2 eq.) under microwave irradiation. In this case, amide (10aA) was isolated in 92% yield by usual work up procedure without employing column chromatography, along with almost recovery of Im-CH2-BtH (6) (98% of its starting material). Thus, the step-wise synthetic strategy leads to the synthesis of amide (10aA) in comparable yields, with or without the isolation of Im-CH2-BtCOPh (Scheme 6).
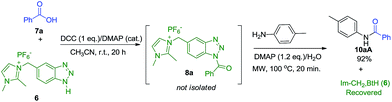 | ||
| Scheme 6 Sequential synthesis of 10aA without isolating imidazolium-supported acyl benzotriazole (8a). | ||
Application of imidazolium-supported acetyl benzotriazole (8h) for the synthesis of paracetamol
Lastly, we applied the chemical utility of imidazolium supported benzotriazole reagent towards the synthesis of paracetamol. Paracetamol (N-(4-hydroxyphenyl)acetamide) is a mild painkiller and reduces the temperature of patients with fever, and thus widely used OTC drug in many countries either in different pharmaceutical formulations, alone or in combination with other active pharmaceutical ingredients. Many synthetic routes have been explored for the paracetamol production but all those involve the acetylation of p-aminophenol using acetic anhydride at the final stage. We attempted to substitute corrosive acetic anhydride by imidazolium-supported N-acetyl benzotriazole 8h for preparing paracetamol in a cleaner and safer way. 8h effectively and selectively acetylated amino group of p-aminophenol (9F) using DMAP in water under microwave irradiation in 15 minutes yielding paracetamol (10hF) in 93% yield, along with the easy recovery of Im-CH2-BtH (6) in pure form as described for other products. In order to evaluate the potential of this process on a larger scale, a one gram batch production of paracetamol from 3.95 g of 8h and 1.0 g of p-aminophenol was successfully achieved under optimized conditions (Scheme 7).Experimental
Materials and methods
All the chemicals were purchased from “Sigma-Aldrich”, Alfa Aesar, and Spectrochem India Pvt. Ltd and were used without further purification. The solvents used were purchased from Merck (India) and were distilled and dried before use. Nuclear magnetic resonance spectra were recorded on Bruker Avance™ 400 spectrometer. All 1H NMR experiments were reported in δ units, parts per million (ppm), and were measured relative to residual chloroform (7.26 ppm) or DMSO (2.5 ppm) in the deuterated solvent. All 13C NMR spectra were reported in ppm relative to deuterochloroform (77.0 ppm) or [D6]-DMSO (39.5 ppm). All coupling constants J were reported in Hz. The following abbreviations were used to describe peak splitting patterns when appropriate: s = singlet, d = doublet, t = triplet, dd = doublet of doublet, m = multiplet and br s = broad singlet. Melting points were determined on a capillary point apparatus equipped with a digital thermometer and are uncorrected. Mass spectra were recorded on an AB SCIEX TOF/TOF 5800 spectrometer. The reactions were carried in a CEM Discover BenchMate reactor in 10 mL pressure vials at 80 W. DSC was recorded on Perkin Elmer DSC 4000 at a heating rate of 10 °C min−1 using nitrogen as the carrier gas in the range 0 °C to 280 °C. Similarly, thermogravimetric analysis (TGA) was obtained using the Perkin Elmer TGA 4000 in the presence of nitrogen flow at a linear heating of 10 °C min−1 starting from 25 °C to 500 °C.Recrystallization in methanol yielded pure N1-isomer of 6. White solid (N1-isomer); m.p: 172–173 °C; 1H NMR (400 MHz, DMSO-d6) δ 15.86 (s, 1H), 7.94 (s, 2H), 7.71 (d, J = 34.9 Hz, 2H), 7.43 (d, J = 7.0 Hz, 1H), 5.57 (s, 2H), 3.76 (s, 3H), 2.63 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 145.20, 140.39, 139.40, 131.61, 125.34, 123.15, 121.66, 116.04, 115.39, 51.15, 35.28, 9.97; HRMS (ESI-TOF) (m/z) calculated for C12H15N5+ 229.1327; found 229.1326 [M + 1 − PF6]+.
3-((1-Benzoyl-1H-benzo[d][1,2,3]triazol-5-yl)methyl)-1,2-dimethyl-1H-imidazole-3-ium hexafluorophosphate (8a). White solid (N1-isomer); yield: 75%; m.p: 164–166 °C; 1H NMR (400 MHz, DMSO-d6 + CDCl3) δ 8.37 (d, J = 8.6 Hz, 1H), 8.25 (s, 1H), 8.16–8.12 (m, 2H), 7.84–7.79 (m, 1H), 7.77 (dd, J = 4.8, 2.6 Hz, 2H), 7.68 (d, J = 2.1 Hz, 1H), 7.65 (t, J = 7.8 Hz, 2H), 5.68 (s, 2H), 3.82 (s, 3H), 2.68 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CDCl3) δ 166.06, 145.43, 133.64, 133.05, 131.30, 130.98, 130.33, 128.27, 122.79, 121.25, 119.21, 114.99, 50.26, 34.84, 9.48; HRMS (ESI-TOF) (m/z) calculated for C19H19N5O+ 333.1589; found 333.1614 [M + 1 − PF6]+.
3-((1-(4-Chlorobenzoyl)-1H-benzo[d][1,2,3]triazol-5-yl)methyl)-1,2-dimethyl-1H-imidazole-3-ium hexafluorophosphate (8b). Brown solid (N1 + N3-isomer); yield: 70%; m.p: 142–146 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.37 (dd, J = 9.8, 6.5 Hz, 1H), 8.27 (s, 1H), 8.15 (d, J = 8.3 Hz, 2H), 7.86–7.80 (m, 1H), 7.76 (d, J = 8.4 Hz, 2H), 7.70 (s, 1H), 7.60 (d, J = 8.3 Hz, 1H), 5.75–5.65 (m, 2H), 3.78 (s, 3H), 2.65 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.01, 145.40, 139.24, 133.77, 131.61, 131.10, 130.57, 129.14, 123.28, 121.75, 121.24, 119.65, 115.54, 114.58, 50.95, 50.59, 35.33, 10.19; HRMS (ESI-TOF) (m/z) calculated for C19H18ClN5O+ 367.1199; found 367.1219 [M + 1 − PF6]+.
3-((1-(3,4-Dimethoxybenzoyl)-1H-benzo[d][1,2,3]triazol-5-yl)methyl)-1,2-dimethyl-1H-imidazole-3-ium (8c). White solid (N1 + N3-isomer); yield: 80%; m.p: 223–227 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.33 (dd, J = 15.0, 6.3 Hz, 1H), 8.28 (d, J = 11.0 Hz, 1H), 7.86 (dd, J = 8.5, 1.8 Hz, 1H), 7.83–7.73 (m, 2H), 7.71 (s, 2H), 7.23 (d, J = 8.7 Hz, 1H), 5.73–5.65 (m, 2H), 3.92 (s, 3H), 3.85 (s, 3H), 3.78 (s, 3H), 2.64 (d, J = 7.3 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 165.66, 154.35, 148.77, 145.75, 145.41, 137.71, 133.74, 132.76, 132.37, 130.82, 127.38, 126.79, 123.36, 123.21, 121.73, 121.11, 119.61, 115.47, 114.47, 111.48, 56.43, 56.18, 50.90, 50.61, 35.34, 9.99; HRMS (ESI-TOF) (m/z) calculated for C21H23N5O3+ 393.1800; found 393.1827 [M + 1 − PF6]+.
1,2-Dimethyl-3-((1-(2-methylbenzoyl)-1H-benzo[d][1,2,3]triazol-5-yl)methyl)-1H-imidazole-3-ium hexafluorophosphate (8d). White solid (N1 + N3-isomer); yield: 72%; m.p: 122–124 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.43–8.23 (m, 2H), 7.83 (dd, J = 17.4, 5.1 Hz, 1H), 7.78–7.64 (m, 3H), 7.59 (t, J = 8.4 Hz, 1H), 7.49–7.38 (m, 2H), 5.74–5.66 (m, 2H), 3.79 (s, 3H), 2.66 (s, 3H), 2.37 (2 s, 3H total, for N1 + N3-isomers); 13C NMR (100 MHz, DMSO-d6) δ 168.09, 146.21, 145.81, 145.47, 138.03, 137.69, 134.05, 132.39, 131.61, 131.21, 130.62, 127.10, 125.93, 121.73, 121.25, 119.74, 115.36, 114.46, 51.06, 50.73, 35.34, 19.84, 10.00, 9.98; HRMS (ESI-TOF) (m/z) calculated for C20H21N5O+ 347.1746; found 347.1764 [M + 1 − PF6]+.
(E)-3-((1-Cinnamoyl-1H-benzo[d][1,2,3]triazol-5-yl)methyl)-1,2-dimethyl-1H-imidazole-3-ium hexafluorophosphate (8e). Brown solid (N1-isomer); yield: 72%; m.p: 214–216 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.36 (d, J = 8.5 Hz, 1H), 8.13 (s, 1H), 8.09 (s, 1H), 8.01 (dd, J = 16.3, 2.9 Hz, 1H), 7.82 (d, J = 3.7 Hz, 1H), 7.72 (dd, J = 27.4, 2.6 Hz, 4H), 7.56 (s, 1H), 7.46 (s, 2H), 5.62 (s, 2H), 3.81 (s, 3H), 2.65 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 163.84, 158.88, 149.04, 146.37, 145.48, 134.22, 133.94, 132.21, 131.17, 129.70, 123.34, 121.68, 119.75, 116.35, 115.39, 50.60, 35.35, 34.60, 10.02; HRMS (ESI-TOF) (m/z) calculated for C21H21N5O+ 359.1746; found 359.1767 [M + 1 − PF6]+.
3-((1-Isonicotinoyl-1H-benzo[d][1,2,3]triazol-5-yl)methyl)-1,2-dimethyl-1H-imidazole-3-ium hexafluorophosphate (8f). White solid (N1 + N3-isomer); yield: 65%; m.p: 181–184 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.23 (s, 1H), 8.92 (d, J = 2.7 Hz, 1H), 8.49 (d, J = 7.5 Hz, 1H), 8.44–8.24 (m, 2H), 7.89–7.58 (m, 4H), 5.75–5.67 (m, 2H), 3.79 (s, 3H), 2.65 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.72, 154.02, 151.91, 146.21, 145.26, 139.47, 138.14, 134.17, 131.87, 131.21, 128.30, 127.19, 123.89, 123.36, 121.74, 121.28, 119.69, 115.47, 114.49, 50.87, 50.59, 35.35, 9.99; HRMS (ESI-TOF) (m/z) calculated for C18H18N6O+ 334.1542; found 334.1561 [M + 1 − PF6]+.
3-((1-(Furan-2-carbonyl)-1H-benzo[d][1,2,3]triazol-5-yl)methyl)-1,2-dimethyl-1H-imidazole-3-ium hexafluorophosphate (8g). White solid (N1 + N3-isomer); yield: 68%; m.p: 220–224 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.34 (dd, J = 13.0, 8.6 Hz, 2H), 8.27 (s, 1H), 8.07 (d, J = 3.5 Hz, 1H), 7.86–7.74 (m, 2H), 7.69 (s, 1H), 6.94 (dd, J = 3.6, 1.6 Hz, 1H), 5.73–5.64 (m, 2H), 3.79 (s, 3H), 2.65 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 154.80, 151.00, 145.65, 145.36, 144.24, 137.97, 133.91, 132.40, 132.00, 131.13, 126.94, 125.63, 123.33, 121.72, 121.26, 119.75, 115.34, 114.42, 113.96, 50.88, 50.59, 35.33, 9.98; HRMS (ESI-TOF) (m/z) calculated for C17H17N5O2+ 323.1382; found 323.1410 [M + 1 − PF6]+.
3-((1-Acetyl-1H-benzo[d][1,2,3]triazol-5-yl)methyl)-1,2-dimethyl-1H-pyrazol-2-ium (8h). White solid (N1-isomer); yield: 85%; m.p: 198–200 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.26 (d, J = 7.5 Hz, 1H), 8.21 (s, 1H), 7.75 (s, 2H), 7.69 (s, 1H), 5.66 (s, 2H), 3.77 (s, 3H), 2.94 (s, 3H), 2.63 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.26, 146.25, 145.85, 145.46, 137.85, 133.64, 130.99, 126.69, 123.33, 121.65, 121.14, 119.59, 115.06, 114.22, 50.70, 35.33, 23.61, 9.97.
For N-nucleophilic substrates, the organic filtrate was washed with 1 N HCl, dried with Na2SO4 and concentrated to yield pure amides 10.
For S-nucleophilic substrates, the organic filtrate was washed with water, concentrated and recrystallized with hexane to yield pure thioesters 12.
For O-nucleophilic substrates, the organic filtrate was washed with 2 N NaOH, dried with Na2SO4 and concentrated to yield pure esters 14.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) as eluent yielded 10aA in 82% yield.
9, v/v) as eluent yielded 10aA in 82% yield.
Conclusions
In summary, we have synthesized and characterized imidazolium-supported benzotriazole reagent as a novel synthetic auxiliary and explored its utility as greener carboxyl group activating reagent for the synthesis of amides, esters and thioesters in aqueous microwave conditions via the formation of stable imidazolium-supported acyl benzotriazoles. The reagent is moisture insensitive and highly stable under thermal and aerobic conditions. The use of an aqueous medium and microwave energy in the acylation reaction, high yield of the amides, esters and thioesters in short reaction times, chromatography free purification and easy recyclability and reusability of imidazolium-supported benzotriazole makes it a greener, economical and environmentally friendly activating reagent. The other applications of this reagent are in progress.Acknowledgements
The authors acknowledge BITS Pilani for providing research grant (BITS Seed Grant) for the work. The authors SMAS thank UGC, New Delhi, India for providing BSR fellowship. The author SC thanks CSIR, New Delhi for senior research fellowship. The author KB acknowledge DST, New Delhi for providing research funding.Notes and references
- A. K. Ghose, V. N. Viswanadhan and J. J. Wendoloski, J. Comb. Chem., 1999, 1, 55 CrossRef CAS.
- T. Nogrady and D. F. Weaver, Medicinal Chemistry: A Molecular and Biochemical Approach, Oxford University Press, 2005 Search PubMed.
- A. Rodriguez-Galan, L. Franco and J. Puiggali, Polymer, 2011, 3, 65 CAS.
- N. M. Nielsen and H. Bundgaard, J. Med. Chem., 1989, 32(3), 727 CrossRef CAS.
- A. K. Bansal, R. K. Khar, R. Dubey and A. K. Sharma, Drug Dev. Ind. Pharm., 2001, 27(1), 63 CrossRef CAS PubMed.
- A. K. Gadad, S. Bhat, V. S. Tegeli and V. V. Redasani, Arzneim.-Forsch., 2002, 52(11), 817 CAS.
- F. Cao, J.-X. Guo, Q.-N. Ping and Z.-G. Liao, Eur. J. Pharm. Sci., 2006, 29(5), 385 CrossRef CAS PubMed.
- K. Kankanala, V. R. Reddy, Y. P. Devi, L. N. Mangamoori, K. Mukkanti and S. Pal, J. Chem., 2013, 2013, 1 CrossRef PubMed.
- (a) P. E. Dawson and S. B. H. Kent, Annu. Rev. Biochem., 2000, 69, 923 CrossRef CAS PubMed; (b) S. B. H. Kent, Chem. Soc. Rev., 2009, 38, 338 RSC; (c) R. J. Clark and D. J. Craik, Biopolymers, 2010, 94, 414 CrossRef CAS PubMed.
- A. R. Katritzky, K. Suzuki and Z. Wang, Synlett, 2005, 11, 1656 CrossRef.
- S. S. Panda, C. D. Hall, E. Scriven and A. R. Katritzky, Aldrichimica Acta, 2013, 46, 43 Search PubMed.
- (a) A. R. Katritzky, H.-Y. He and K. Suzuki, J. Org. Chem., 2000, 65, 8210 CrossRef CAS PubMed; (b) A. R. Katritzky, A. A. Shestopalov and K. Suzuki, Synthesis, 2004, 1806 CrossRef CAS; (c) Y. W. Tu, L. J. Zhou, X. Lv and X. X. Wang, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2014, 53, 435 Search PubMed; (d) S.-M. Lin, J.-L. Zhang, J.-X. Chen, W.-X. Gao, J.-C. Ding, W.-K. Sua and H.-Y. Wu, J. Braz. Chem. Soc., 2010, 21(9), 1616 CrossRef CAS; (e) A. R. Katritzky, S. Hoffmann and K. Suzuki, ARKIVOC, 2004, xii, 14 Search PubMed.
- (a) A. Paio, A. Zaramella, R. Ferritto, N. Conti, C. Marchioro and P. Seneci, J. Comb. Chem., 1999, 1(4), 317 CrossRef CAS; (b) K. Schiemann and H. D. H. Showalter, J. Org. Chem., 1999, 64, 4972 CrossRef CAS; (c) S. Talukdar, R.-J. Chen, C.-T. Chen, L.-C. Lo and J.-M. Fang, J. Comb. Chem., 2001, 3(4), 341 CrossRef CAS PubMed; (d) A. R. Katritzky, A. Pastor, M. Voronkov and D. Tymoshenko, J. Comb. Chem., 2001, 3, 167 CrossRef CAS PubMed; (e) A. R. Katritzky, S. A. Belyakov and D. O. Tymoshenko, J. Comb. Chem., 1999, 1, 173 CrossRef CAS.
- Y. Peng, F. Yi, G. Song and Y. Zhang, Monatsh. Chem., 2005, 136, 1751 CrossRef CAS; J. Fraga-Dubreuil and J. P. Bazureau, Tetrahedron, 2003, 59, 6121 CrossRef; W. Miao and T. H. Chan, Org. Lett., 2003, 5, 5003 CrossRef PubMed; S. Anjaiah, S. Chandrasekhar and R. Gree, Tetrahedron Lett., 2004, 45, 569 CrossRef PubMed; M. de Kort, A. W. Tuin, S. Kuiper, H. S. Overkleeft, G. A. van der Marelb and R. C. Buijsmana, Tetrahedron Lett., 2004, 45, 2171 CrossRef PubMed.
- (a) J. L. Anthony, E. J. Maginn and J. F. Brennecke, J. Phys. Chem. B, 2002, 106, 7315 CrossRef CAS; (b) A. Boesmann, L. Datsevich, A. Jess, A. Lauter, C. Schmitz and P. Wasserscheid, Chem. Commun., 2001, 2494 RSC.
- (a) W. Miao and T. H. Chan, Acc. Chem. Res., 2006, 39(12), 897 CrossRef CAS PubMed; (b) M. Pucheault and M. Vaultier, Top. Curr. Chem., 2010, 290, 83 CrossRef.
- (a) J. Vitz, D. H. Mac and S. Legoupy, Green Chem., 2007, 9, 431 RSC; (b) K. Pandey, M. K. Muthyala, S. Choudhary and A. Kumar, RSC Adv., 2015, 5, 13797 RSC.
- X. He and T. H. Chan, Org. Lett., 2007, 9(14), 2681 CrossRef CAS PubMed; S.-Y. Han and Y.-A. Kim, Tetrahedron, 2004, 60, 2447 CrossRef PubMed.
- C. K. Yerneni, V. Pathak and A. K. Pathak, J. Org. Chem., 2009, 74(16), 6307 CrossRef CAS PubMed.
- A. R. Katritzky, F.-B. Ji and W.-Q. Fan, Synth. Commun., 1993, 23(14), 2019 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to late Prof. A. R. Katritzky for his contribution to benzotriazole chemistry. |
| ‡ Electronic supplementary information (ESI) available: 1H & 13C NMR spectra of 4–6 and 8a–h. Characterization data and 1H NMR & 13C NMR spectra of 10aA–10hF, 12aA–12gB, 14aA–14gB. See DOI: 10.1039/c5ra18749d |
| This journal is © The Royal Society of Chemistry 2015 |