Synthesis of a novel dendrimer core of oxo-vanadium phthalocyanine magnetic nano particles: as an efficient catalyst for the synthesis of 3,4-dihydropyrano[c]chromenes derivatives under green condition†
Maliheh Safaiee*a,
Mohammad Ali Zolfigol*b,
Fatemeh Afsharnaderyb and
Saeed Bagheryb
aDepartment of Medicinal Plants Production, Nahavand University, Nahavand, 6593139565, Iran. E-mail: azalia_s@yahoo.com; msafaiee@nahgu.ac.ir
bDepartment of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, Hamedan 6517838683, Iran. E-mail: zolfi@basu.ac.ir; mzolfigol@yahoo.com
First published on 20th November 2015
Abstract
A new magnetically recoverable nanocatalyst was synthesized by covalent binding of an amino vanadium oxo phthalocyanine, on the surface of silica coated magnetite nano particles with 3-chloropropyl moieties. Full characterization of the prepared nano catalyst was performed with different physicochemical methods such as FT-IR spectra, X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscope (AFM), and thermal gravimetric analysis (TGA), energy-dispersive X-ray spectroscopy (EDX), and vibrating sample magnetometry (VSM), Brunauer–Emmett–Teller (BET), X-ray fluorescence (XRF) and inductively coupled plasma (ICP). Finally, catalytic activity of the prepared Fe3O4@SiO2@SiO2(CH2)3@AVOPc was examined in the synthesis of 3,4-dihydropyrano[c]chromenes derivatives. The nano catalysts provided excellent catalytic activities to yield the desired products in short reaction time, mild and green conditions under solvent-free at room temperature. The catalysts could be easily separated from the reaction mixture by a magnet, and recycled eight consecutive cycles without any losing significant activities. Moreover, by this synthetic method, some novel 3,4-dihydropyrano[c]chromenes derivatives are prepared and characterized.
Introduction
The preparation and the use of nanoparticles (NPs) in organic synthesis has become a subject of intense investigation. In particular, magnetic nanoparticles (MNPs) which offer advantages in clean and sustainable chemistry as they can be non-toxic, readily available, and recoverable.1 Therefore design of novel nano magnetically separable catalytic systems have attracted attention in recent times as an interesting alternative to improve the efficient separation of heterogeneous (nano) catalysts from solutions over reaction completion by applying a simple magnet, providing improved recyclability in the designed systems.2 Among magnetic nanoparticles (MNPs), nano magnetic core–shell structures are highly applied in bio and environmental researches. Magnetic core–shell, consisting of an iron oxide core and a silica shell, has attracted more consideration for their unique magnetic properties, good stability, low cytotoxicity, and chemically modifiable surface.3Phthalocyanines (Pcs) find versatile applications in the area of material science because of their specific optical and electrical properties with suitable chemical and thermal stability.4 Pcs are used in a number of applications due to their increased stability, architectural designing flexibility, various coordination properties and improved spectroscopic characteristics.5 The two hydrogen atoms of the central cavity could be replaced by more than 70 central metals and a wide variety of substituents could be incorporated, namely the metal phthalocyanine molecules (MPc) which display interesting and useful optical, catalytic, electronic, and biological properties.6
Metallophthalocyanines have been used as efficient biomimetic catalysts for oxidation, reduction and other functional groups transformations.7 Vanadium phthalocyanines have applied in photoelectrophoretic and optical recording materials8 and have been also used for polymerisation of ethylene in the presence of methylaluminoxane (MAO) as a co-catalyst.9 Therefore, immobilization of the metal phthalocyanine via covalent attachment to the nano magnetic core–shell is an interesting approach to facilitate catalyst recovery, recycling, and to reduce effluent contamination. In addition, these immobilized catalysts resistant to leaching due to the chemical bonding between the support and the metal phthalocyanine. Most importantly, a MNPs supported catalyst can be conveniently separated from the final reaction mixture upon completion by using an external magnet without filtration.10
Dihydropyrano[c]chromenes and their derivatives possess a wide range of biological properties11 such as anti-cancer, anticoagulant, diuretic, and anti-anaphylactic activities.12 Literature surveys shows that, few methods have been reported for the synthesis of dihydropyrano[c]chromene derivatives. Some of these compounds have been already synthesized in the presence of S-proline and diammonium hydrogen phosphate (DAHP),13 K2CO3 under microwave irradiation,14 H6P2W18O62·18H2O,15 MgO,16 tetrabutylammonium bromide (TBAB),17 sodium dodecyl sulfate,18 DBU,19 morpholine,20 α-Fe2O3 nanopatricles,21 CuO nanoparticles,22 and silica-bonded N-propyl-piperazine sodium propionate.23
In continuation of our studies on the synthesis of solid acids,24 inorganic acidic salts,25 nano magnetic catalysts2,26 and knowledge-based development of task specific phthalocyanines27 we decided to join all of these research areas to design, synthesis and applications of Fe3O4@SiO2@SiO2(CH2)3@AVOPc. In this work, our objective is to functionalize magnetite nanoparticles with 3-chloropropyl moieties and then incorporate a oxo-vanadium phthalocyanine with anchoring method to obtain a nano heterogeneous oxo-vanadium phthalocyanine catalyst for the synthesis of 3,4-dihydropyrano[c]chromenes derivatives.
Results and discussion
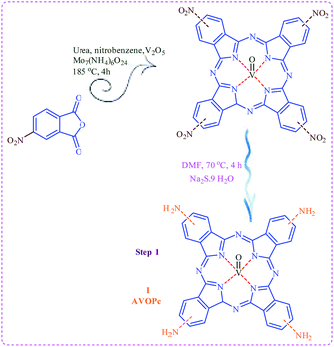
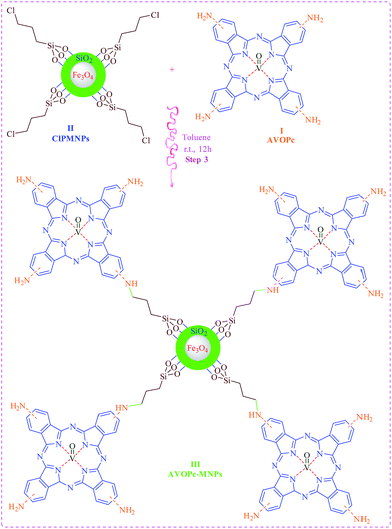
Knowledge-based development of task specific magnetic nano particles catalysts and their structural diversity could be achieved via designing and synthesis of novel cores with suitable active sites. By considering the above-mentioned synthetic strategy, in the course of our investigations on designing and synthesis of heterogeneous catalysts2,24–27 lead us to report Fe3O4@SiO2@SiO2(CH2)3@AVOPc as a novel magnetic nano particles catalyst with functionalized phthalocyanine moieties. Synthesis of the catalyst consists of three following stages.(1) In the first step synthesis of the amino oxo-vanadium phthalocyanine takes place (as shown the first step of Scheme 1 step 1).
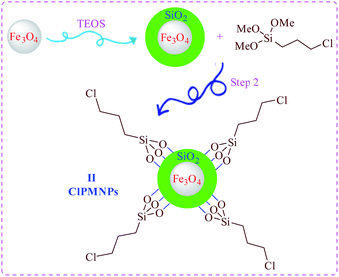
(2) Nano magnetic particles core was synthesized in the second step (as depicted in Scheme 2 step 2).
(3) Finally nano magnetite and oxo vanadium were obtained from step 1 and 2 combine with each other to synthesis of final catalyst.
Initially, to prepare the amino oxo-vanadium phthalocyanine (AVOPc), 3-nitro phthalic anhydride species must be reflux with the urea, vanadium oxide, ammonium molybdate.28 The reaction mixture was cooled and diluted with toluene. The resulting precipitate was collected by centrifugation. The obtained solid was washed with toluene, water, MeOH/ether, EtOAc/hexane, and dried to afford a dark green solid. After that to reduce nitro to amino, sodium sulfide nine hydrates was added to a solution of tetra nitro oxo-vanadium phthalocyanine (NVOPc). Resulting precipitate collected by centrifugation (Scheme 1).
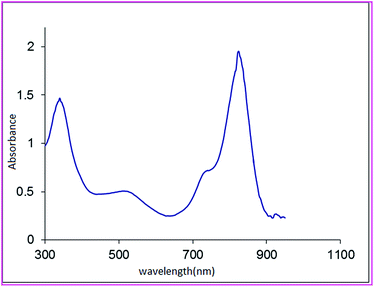
The AVOPc was characterised using IR and UV-visible. UV-visible spectra of VOPc complexes in freshly distilled DMF are shown in Fig. 1.
The UV-visible spectra consist of the three absorption bands.
(i) The Q band is observed at 822 nm in DMF. In AVOPc Q band is shifted out of the visible region into the near infrared region so that the color of the synthesized complex is dark green.29
The large red shift of the Q band is due to the large electron-donating ability of the amino groups.
(ii) Weaker band in the region of 337 nm called B or the Soret band.
(iii) Charge transfer transitions located between the Q and B bands. A broad band between 450 and 600 is probably due to charge transfer.
For the synthesis of the material of step 2, MNPs (Fe3O4) was prepared according the previously reported method. And subsequently coated with a layer of silica upon reaction with tetraethyl orthosilicate (TEOS).30 Chloro functionalized MNPs (II) (Scheme 2, step 2) were then synthesized by adding silica coated MNPs to a solvent mixture consisting of DMF and toluene, followed by stirring.31
(3-Chloropropyl)trimethoxysilane [CPTMS] was then added drop-wise to the mixture using a syringe. The mixture was stirred for 24 h at room temperature. The as-prepared 3-chloropropyl coated MNPs (II) Fe3O4@SiO2@SiO2(CH2)3Cl (ClPMNPs) were washed four times with toluene and collected with the aid of a magnet.
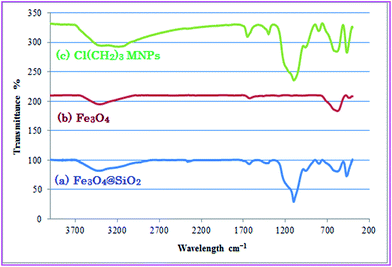
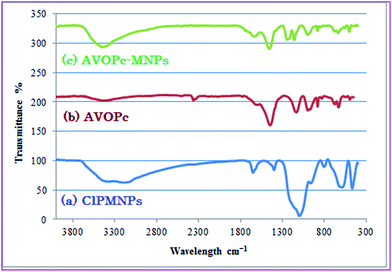
The Fourier transforms infrared (FTIR) spectra of (a) Fe3O4@SiO2, (b) Fe3O4 and (c) Fe3O4@SiO2 modified by CPTMS is shown in Fig. 2. In Fig. 2b, the band at 592 cm−1 is related to the Fe–O bending vibration. The silica coating of magnetite nanoparticles was confirmed by observation of high-intensity band about 1098 cm−1 assigned to asymmetric stretching bonds of Si–O–Si and Si–OH stretching vibrations. The broad peaks in the range 3100–3600 cm−1 and the peak at 1632 cm−1 are assigned to the O–H stretching vibration mode of Si–OH and twisting vibration mode of H–O–H adsorbed in the silica shell, respectively.32
The silica adsorbed on the magnetite surface by Fe–O–Si bonds cannot be seen in the FTIR spectrum because it appears at around 592 cm−1 and overlaps with the Fe–O vibration of magnetite nanoparticles.
In Fig. 2c, the presence of anchored propyl spacer were confirmed by stretching vibrations appeared at about 2900–3000 cm−1 in the FT-IR.
In the next step for covalently linking the ClPMNPs to AVOPc, chloro functionalized MNPs and toluene were sonicated and subsequently added to the AVOPc. The mixture was stirred for 12 h at room temperature. The product was precipitated by addition of ethanol, and washed with ethanol followed by centrifugation. A magnet was also used to separate the Fe3O4@SiO2@SiO2(CH2)3Cl@AVOPc (AVOPc-MNPs) from unreacted Pc (Scheme 3).
The FT-IR spectroscopy indicated the successful synthesis of AVOPc. The absorption at 1611 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and 1445 (C
N) and 1445 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) cm−1 were observed for AVOPc. The other absorption peaks at 3442 and 997 cm−1 could be assigned to NH2 stretching and V
C) cm−1 were observed for AVOPc. The other absorption peaks at 3442 and 997 cm−1 could be assigned to NH2 stretching and V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching due to the presence of amino and vanadium oxo groups in AVOPc (Fig. 3a).
O stretching due to the presence of amino and vanadium oxo groups in AVOPc (Fig. 3a).
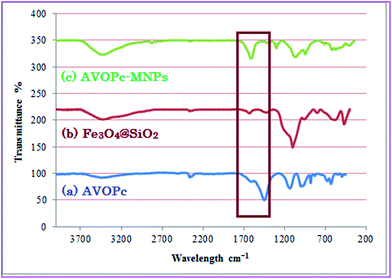 | ||
| Fig. 3 FT-IR spectra of (a) AVOPc (b) Fe3O4@SiO2 (c) AVOPc-MNPs (mixed one pot of AVOPc, Fe3O4@SiO2 and CPTMs). | ||
In order to facilitate the reaction, CPTMS, VOPc and MNPs@SiO2 were reflux for 24 hours in toluene and the product was collected with the aid of a magnet but this is not an appropriate method for the synthesis of AVOPc-MNPs because in the FT-IR spectrum some strong bands at 1448 cm−1 assigned to stretching vibrations of aromatic rings of AVOPc ligand were observed that were not present in the prepared catalyst (Fig. 3).
Immobilization of the AVOPc on ClPMNPs was confirmed by FT-IR spectroscopy (Fig. 4c). For the AVOPc-MNPs characteristic strong band are seen at 1635 and 1457 cm−1 which did not observe for ClPMNPs and indicated that phthalocyanine immobilized on nano magnetite core.
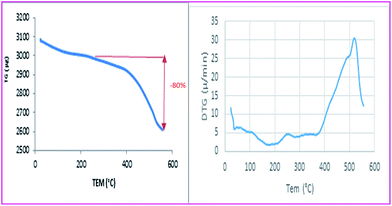
Thermal analysis of the catalyst gives information about the stability of the VOPc-MNPs catalyst. In the TG curve of catalyst, the weight loss at temperatures below 250 °C is due to the removal of physically adsorbed solvent and surface hydroxyl groups.33
In the second step, at about 260 °C to nearly 555 °C is attributed to the decomposition of the coating organic layer in the nano composite. Therefore, the weight loss between 260–555 °C gives the organic grafting ratios to the magnetic catalyst. The grafted AVOPcs on the magnetic Fe3O4@SiO2 nanoparticles was approximately 80 wt%. This mass loss of organic components is equal to 1.17 mmol per g of catalyst (Fig. 5). Furthermore, the DTG curve shows that the decomposition of the organic structure mainly occurred at 530 °C. Analysis of these diagrams strongly proposed that phthalocyanine structure was stable and no further weight loss occurs below 400 °C therefore high thermal stability of the catalyst indicated covalent chemical bonds between amino phthalocyanine and supporter.
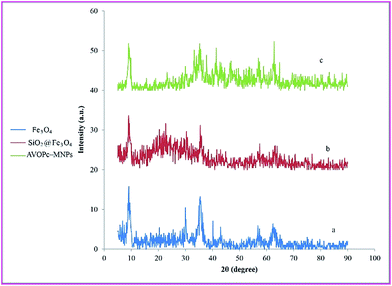
The X-ray diffraction patterns of MNPs, MNPs@SiO2 and AVOPc-MNPs are shown in Fig. 6. XRD diffraction peaks at 2θ = 30.4°, 35.7°, 43.3°, 53.9°, 57.3° and 63.0° indicating that the Fe3O4 particles in the nanoparticles were pure Fe3O4 with a cubic spinel structure. The broad peak from 2θ = 20° to 27° (Fig. 6b) is indicated an amorphous silica phase in the shell of the silica-coated Fe3O4 nanoparticles (Fe3O4@SiO2). In Fig. 6c, we can observe that the XRD pattern of the AVOPc-MNPs is similar to the pattern of Fe3O4@SiO2 nanoparticles because the coating of the AVOPc layer does not change the structure of the Fe3O4@SiO2 nanoparticles.34 The average size of the catalyst was calculated by Scherrer's equation from the XRD results, D = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, from the width of the peak at 2θ = 62.8° the crystallite size of the magnetic nanoparticle is calculated to be 50.74 nm in good agreement with observed result in the TEM studies.
θ, from the width of the peak at 2θ = 62.8° the crystallite size of the magnetic nanoparticle is calculated to be 50.74 nm in good agreement with observed result in the TEM studies.
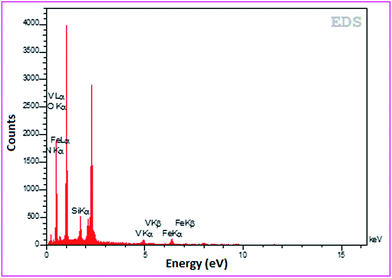
The EDX spectrum shows the kinds of elements present (V, O, Fe, Si and N) in the AVOPc-MNPs and no other impurities occur (Fig. 7).
XRF analysis of the catalyst showed that the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O content was 4.74 wt% in the catalyst. The specific surface area of the catalyst was calculated from adsorption isotherms using the standard BET equation. The Brunauer–Emmett–Teller (BET) surface area calculated 9.26 m2 g−1.
O content was 4.74 wt% in the catalyst. The specific surface area of the catalyst was calculated from adsorption isotherms using the standard BET equation. The Brunauer–Emmett–Teller (BET) surface area calculated 9.26 m2 g−1.
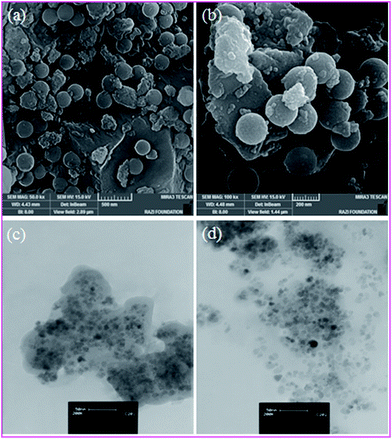
To determine the morphology and the size of the catalyst, SEM and TEM were used. This image of the catalyst shows that these particles have nearly spherical shape with sizes about 50 nm (Fig. 8).
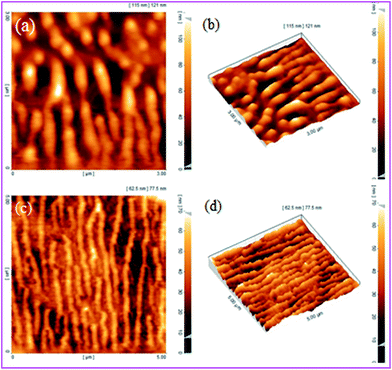
Atomic force microscopy (AFM) is a technique that lets us find and analyze surfaces with high resolution and consideration. AFM affords abundant benefits; actually any classic can be imaged: for example hard surfaces for instance the surface of a ceramic material, or the dispersal of metallic nano composite; or very soft, such as molecules of proteins or plastic materials. Fig. 9 shows the three-dimensional AFM images of AVOPc-MNPs as a catalyst. No significant partition area in size is recognized in the illustrations. Afterward the two- and three-dimensional 2.1 μm2 × 2.1 μm2 frameworks, we can understand that the achieved AVOPc-MNPs catalyst approvals an interrupted structure with a desirable outside planarity. The surface of the coat on the AVOPc-MNPs catalyst was visibly revealed to be less than 50 nm (Fig. 9).
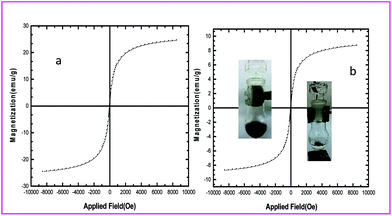
The magnetic property of the MNPs@SiO2 and AVOPc-MNPs were characterized by VSM. The room temperature magnetization curves of the MNPs@SiO2 and the AVOPc-MNPs are shown in Fig. 10a and b. MNPs@SiO2, exhibited super paramagnetic properties with saturation magnetization about 25 emu g−1 and saturation magnetization of AVOPc-MNPs was 8.7 emu g−1. Compared with the MNPs@SiO2 particles, the saturation magnetization of the AVOPc-MNPs obviously decreased because the diamagnetic contribution of the thick CPTMS and organic matter resulted in a low mass fraction of the MNPs@SiO2 magnetic substance. Even with this reduction in the saturation magnetization, the solid could still be efficiently separated from solution with a permanent magnet.
At the beginning of the research, we made a conscious effort to develop a heterogeneous system to synthesis of 3,4-dihydropyrano[c]chromenes. The condensation reaction of malononitrile, 2,5-dimethoxybenzaldehyde, and 4-hydroxycoumarin was chosen as a model reaction (Scheme 4).
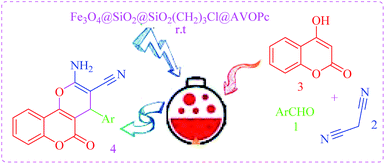 | ||
| Scheme 4 AVOPc-MNPs catalyzed synthesis of 2-amino-4-aryl-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile derivatives. | ||
In order to optimize the reaction conditions and obtain the best catalytic activity, the reaction of 2,5-dimethoxybenzaldehyde (1 mmol), malononitrile (1 mmol), and 4-hydroxycoumarin (1 mmol) were used as a model, and was conducted under different reaction parameters such as solvent, temperature and amount of catalyst. The effect of catalyst amount was firstly investigated and different quantities of the catalyst ranging from 0.02 g to 0.5 g were tested. The best results were obtained using 0.02 g of the catalyst (Table 1). In the next step the model reaction was carried out in several solvents such as EtOH, H2O, CH3CN, CH2Cl2, EtOAc, and under solvent-free conditions (Table 2).
| Entry | Amount of catalyst (g) | Temp (°C) | Time (min) | Isolated yielda (%) |
|---|---|---|---|---|
| a Reaction conditions: 2,5-dimethoxybenzaldehyde (1 mmol), malononitrile (1 mmol), 4-hydroxycoumarin (1 mmol) under solvent free condition. | ||||
| 1 | — | r.t. | 60 | 30 |
| 2 | — | 100 | 60 | 45 |
| 3 | 0.02 | r.t. | 20 | 92 |
| 4 | 0.02 | 75 | 20 | 92 |
| 5 | 0.02 | 100 | 20 | 92 |
| 6 | 0.05 | r.t. | 20 | 92 |
| 7 | 0.05 | 100 | 20 | 92 |
| 8 | 0.1 | r.t. | 20 | 91 |
| 9 | 0.1 | 100 | 20 | 91 |
| 10 | 0.2 | r.t. | 25 | 90 |
| 11 | 0.5 | r.t. | 25 | 90 |
The reaction was efficiently performed using 0.02 g of the nano catalyst at room temperature under solvent free condition to give desired product in high yield within short reaction time (Table 2, entry 1).
A series of 3,4-dihydropyrano[c]chromene derivatives were prepared successfully from different aromatic aldehydes bearing electron-withdrawing and electron-donating groups, 4-hydroxycoumarin, and malononitrile at room temperature under solvent free conditions. Aldehydes bearing electron-withdrawing groups, 3-nitro-, 4-nitro-, and 4-CN–benzaldehyde reacted under the optimized conditions to give 3,4-dihydropyrano[c]chromenes derivatives in 94%, 95%, and 95% yields, respectively after 10–15 min (Table 3, entries 14–16). Aromatic aldehydes possessing electron donating substituents such as 4-methyl, 4-hydroxy, 3-hydroxy, 2-hydroxy, 4-methoxy, 3,4-dimethoxy, 3-ethoxy-4-hydroxyl, 3-methoxy 2-hydroxyl, 4-dimethylamino and 2,5-dimethoxy have afforded the pyranochromenes (87–95% yields) (Table 3, entries 1–5 and 8–11). As shown in Table 3, when halogen substituted aromatic aldehydes such as 4-chloro, 2,4-dichloro, substrates were employed well under the reaction conditions (91–94%) (Table 3, entries 12 and 13). These results clearly indicate that the reactions can tolerate a wide range of differently substituted aldehydes and heterocyclic aldehyde.
| Entry | Aldehyde | Time (min) | Isolated yielda (%) | M.p (°C) |
|---|---|---|---|---|
| a Reaction conditions: aldehyde (1 mmol), malononitrile (1 mmol), and 4-hydroxycoumarin (1 mmol), solvent free condition at room temperature. | ||||
| 1 | 4-Me–C6H4– | 22 | 92 | 255–257 (White) |
| 2 | 4-OMe–C6H4– | 20 | 93 | 235–237 (White) |
| 3 | 4-OH–C6H4– | 25 | 90 | 260–262 (White) |
| 4 | 3-OH–C6H4– | 30 | 88 | 248–250 (White) |
| 5 | 2-OH–C6H4– | 30 | 87 | 271–273 (Yellow) |
| 6 | Cinnamaldehyde | 40 | 81 | 187–189 (Yellow) |
| 7 | C6H5– | 30 | 87 | 245–247 (White) |
| 8 | 4-NMe2–C6H4– | 20 | 89 | 265–267 (White) |
| 9 | 2,5-(MeO)2–C6H3– | 20 | 92 | 258–260 (White) |
| 10 | 3,4-(MeO)2–C6H3– | 20 | 92 | 217–219 (White) |
| 11 | 3-OEt-4-OH–C6H3– | 25 | 95 | 248–250 (White) |
| 12 | 4-Cl–C6H4– | 15 | 94 | 268–271 (White) |
| 13 | 2,4-(Cl)2–C6H3– | 20 | 91 | 258–260 (White) |
| 14 | 4-O2N–C6H4– | 10 | 95 | 260–262 (Yellow) |
| 15 | 3-O2N–C6H4– | 15 | 94 | 262–264 (White) |
| 16 | 4-CN–C6H4– | 12 | 95 | 261–263 (White) |
| 17 | 4-CHO–C6H4– | 25 | 90 | 296–298 (White) |
| 18 | 4-Pyridyl | 35 | 85 | 272–274 (Brown) |
| 19 | 2-Thunyl | 35 | 85 | 245–247 (White) |
| 20 | 2-Furyl | 35 | 85 | 223–225 (Cream) |
The possibility of recycling the catalyst was examined using the model reaction under the optimized conditions. Upon completion the reaction, catalyst collected with the aid of a magnet and washed with hot ethanol, dried in air, and the catalyst was reused in the next reaction. The recycled catalyst could be reused eight times without any appreciable loss of its initial catalytic activity. Results depicted in Fig. 11. The deactivation of the catalyst is low, while reactant was predicted. The reaction was scaled up to 10 mmol of 4-chlorobenzaldehyde, 4-hydroxycoumarin and malononitrile and using 0.2 g of catalyst at room temperature. The yield of the reaction was 94% after 15 min and 87% after the eighth run. The results were summarized in Fig. 11.
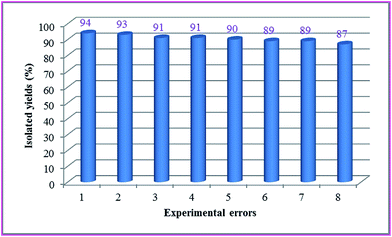 | ||
| Fig. 11 The catalytic activity of AVOPc-MNPs in eighth cycles for the synthesis of 2-amino-4-aryl-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile derivatives. | ||
A possible mechanism for the reaction using AVOPc-MNPs as a clean catalyst is shown in Scheme 5. Initially, according to the mechanism AVOPc-MNPs catalyzed the readily in situ formation of Knoevenagel intermediate I (AVOPc-MNPs) as a catalyst activates the carbonyl group of the aromatic aldehyde. The Knoevenagel condensation of aromatic aldehyde and malononitrile was occurred to form the arylidene malononitrile (I). Formerly, 4-hydroxycoumarin II tolerates nucleophilic attack to arylidene malononitrile I and gives the III (we believe that the AVOPc-MNPs activates the cyanide group of arylidene malononitrile I for nucleophilic attack by 4-hydroxycoumarin (II) to form Michael adduct (III) which undergoes ring closing reaction to form desire product).35 The Michael adduct III tautomerizes and cyclizes using AVOPc-MNPs catalyst to create intermediate IV and which then tautomerized to give the completely aromatized 2-amino-5-oxo-4-aryl-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile.
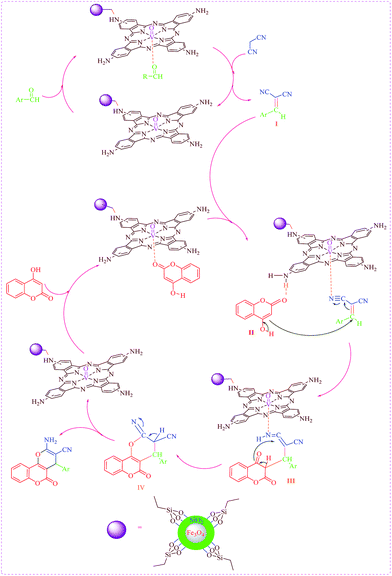 | ||
| Scheme 5 Possible mechanism for one-pot synthesis of 2-amino-5-oxo-4-aryl-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile. | ||
Also, in this study inductively coupled plasma (ICP) spectroscopy was used for the determination of the amount of vanadium in the recycled catalyst. Studies of ICP obtained data show that the amount of vanadium was decreased in the recycled catalyst from 2.20 mg L−1 (in the first run of recycle) to less than 0.1 mg L−1 in the eighth run (which represents leaching of catalyst is decreased in the course of recycling process) (Table 4).
| Experimental errors | Amount of V (mg L−1) |
|---|---|
| 1 | 2.20 |
| 2 | 0.82 |
| 3 | 0.64 |
| 4 | 0.60 |
| 5 | 0.37 |
| 6 | <0.1 |
| 7 | <0.1 |
| 8 | <0.1 |
To compare the efficacy of AVOPc-MNPs catalyst with some reported catalysts for the synthesis of 2-amino-5-oxo-4-aryl-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile, we have presented the results of those reported catalysts to achieve the condensation of 4-chlorobenzaldehyde, 4-hydroxycoumarin and malononitrile, in Table 5. As Table 5 shows, AVOPc-MNPs catalyst has uniquely enhanced the synthesis of desired product in different terms (reaction time and isolated yield).
| Reaction condition | Catalyst loading | Time (min) | Isolated yielda % | Ref. |
|---|---|---|---|---|
| a Isolated yield. | ||||
| [Sipim]HSO4, solvent-free, 100 °C | 0.08 mmol | 30 | 90 | 11 |
| SDS, H2O, 60 °C | 20 mol% | 180 | 96 | 18 |
TBBDA, EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), reflux 1), reflux |
0.18 mmol | 170 | 91 | 36a |
PBBS, EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), reflux 1), reflux |
0.1 g | 150 | 90 | 36a |
| Polymer supported sulphanilic acid, solvent-free, MW | 5% w/w | 90 | 92 | 36b |
| Nano Al2O3, EtOH, r.t. | 25 mol% | 300 | 89 | 36c |
| Starch solution, 50 °C | 4 mL | 45 | 95 | 36d |
| TMGT, 100 °C | 0.1 mmol | 25 | 81 | 36e |
| AVOPc-MNPs catalyst, r.t. (this work) | 0.02 g | 10 | 95 | — |
Experimental
The materials were purchased from Merck and Fluka and were used without any additional purification. All reactions were monitored by thin layer chromatography (TLC) on gel F254 plates. Spectrometer (1H NMR 400 MHz and 13C NMR 100) in pure deuterated DMSO with tetramethylsilane (TMS) as the internal standard. The synthesized catalyst was characterized by FT-IR, XRD, SEM, TEM, AFM, TGA, VSM, XRF, BET and elemental analysis. X-ray diffraction (XRD) patterns of all catalysts were performed on a APD 2000, Ital structure with Cu K_ radiation (k = 0.1542 nm) operating at 50 kV and 20 mA in a 2 h range of 10–70° with step size 0.01° and time step 1.0 s to assess the crystallinity of the catalyst. The vanadium concentration of the prepared catalysts was determined by XRF using oxford instruments. Fourier transform-infrared spectra of the samples were recorded on a Perkin–Elmer FT-IR spectrometer 17259 using KBr disks. Thermogravimetric analyses using a Perkin–Elmer TGA were performed on catalysts. The weight loss between 200 and 600 °C was determined. Semi-quantitative EDX (Röntec, Quantax/QX2) analysis was used for the characterization of element concentration and vanadium distribution within prepared catalysts. The SEM analyses were done with a TESCAN/MIRA with a maximum acceleration voltage of the primary electrons between 10 and 15 kV. Transmission electron microscope, TEM measurements were carried out on a Philips CM10 analyzer. Operating at 120 kV. The magnetization and hysteresis loop were measured at room temperature using a Vibrating Sample Magnetometer (magnetite daneshpazhohan kashan/MDKB) system. The AFM images were obtained in ambient air using a dual scope (Cme/C26) in the constant-force mode at various scan rates. The surface areas of the catalysts (BET) were measured by means of nitrogen physisorption using a Quantachrome Nova Station B instrument. Determination of vanadium concentration was carried out via an inductively Coupled Plasma (ICP) [Model Agilent 7300 DV, Optima, America] in a test standard requirements of ISO/IEC 17025: 2005.General procedure for the synthesis of magnetite nanoparticles
FeCl3·6H2O (9.72 g, 0.036 mol) was added to an aqueous solution of HCl 3% (v/v) and stirred for 10 min with mechanical stirrer (mixture 1). Na2SO3 (2.52 g, 0.0199 mol) dissolved in 20 mL deionized water until the salts dissolved completely (mixture 2). Mixture 2 was added to mixture 1 drop-wise in 2 minutes to obtain a red solution then the mixture stirred for ten minutes to gain the yellow one (mixture 3). Then, 85 mL of ammonia dissolved in 800 mL deionized water. The resulting solution was added quickly into the reaction mixture 3 in one portion to obtain the black solution. The catalyst was removed from the solution by using a magnet. The black precipitate was washed with doubly distilled water (seven times) and ethanol (three times) and dried in the air.Preparation of Fe3O4@SiO2 core–shell
The synthesized MNPs Fe3O4 (2 g) suspended in distilled water (40 mL) and sonicated for 20 min. Ethanol (16 mL), ammonia (5 mL), and tetraethyl orthosilicate (7 mL) were respectively added into the suspension, and continuously reacted for 12 h under stirring at room temperature. The iron oxide nanoparticles with a thin layer of silica (Fe3O4@SiO2) were separated by an external magnet, washed three times with ethanol and water and dried under vacuum.Preparation of chloropropyl functionalized MNPs (ClPMNPs)
Chloropropyl functionalized MNPs were synthesized by adding silica coated MNPs (10 mg) to a solvent mixture consisting of 12 mL DMF and 8 mL toluene, followed by stirring. CPTMS (0.3 mL) was then added drop-wise to the mixture using a syringe. The mixture was stirred for 24 h at room temperature. The chloropropyl coated MNPs (brown powder) were washed four times with toluene and collected by using a magnet.General procedure for synthesis of AVOPc
Ammonium molybdate (0.01 mg) was added to a solution of 3-nitrophthalic anhydride (1.93 g, 10 mmol), urea (3.0 g, 50 mmol), and vanadium pentaoxide (0.473 mg, 2.6 mmol) in nitrobenzene (15 mL). The mixture was stirred under N2 at 200 °C. After 4 h, the reaction mixture was cooled and diluted with toluene (80 mL). The resulting precipitate was collected by centrifugation. The solid was washed with toluene, water, MeOH/ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), EtOAc/hexane (2
9), EtOAc/hexane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and dried to afford a dark green solid (2.56 g, 99%). After that sodium sulfide nine hydrates (7.4 g, 30.9 mmol) was added to tetranitro vanadium-oxo phthalocyanine (1.95 g, 2.57 mmol) in DMF (50 mL) and stirred at 70 °C for 4 h. After complication of reaction mixture was cooled and collected by centrifugation. The product was washed by addition of methanol/ether (1
1), and dried to afford a dark green solid (2.56 g, 99%). After that sodium sulfide nine hydrates (7.4 g, 30.9 mmol) was added to tetranitro vanadium-oxo phthalocyanine (1.95 g, 2.57 mmol) in DMF (50 mL) and stirred at 70 °C for 4 h. After complication of reaction mixture was cooled and collected by centrifugation. The product was washed by addition of methanol/ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), methanol and washed two times with ethanol followed by centrifugation. The mixture dried to obtain a brown solid phthalocyanine.
9), methanol and washed two times with ethanol followed by centrifugation. The mixture dried to obtain a brown solid phthalocyanine.
Preparation of AVOPc-MNPs
Chloropropyl functionalized MNPs (5 mg) was suspended in 5 mL of toluene with sonication. To this mixture was added AVOPc (15 mg), and the resulted mixture was stirred for 12 h. The product was precipitated by addition of ethanol, and washed with ethanol followed by centrifugation. A magnet was also used to separate the final catalyst and dried in the air.General procedure for the synthesis of dihydropyrano[c]chromene derivatives
A solution of 4-hydroxycoumarin (0.162 g, 1 mmol), aromatic aldehydes (1 mmol), malononitrile (0.066 g, 1 mmol) and AVOPc-MNPs (0.02 g) was stirred at room temperature for required time (Table 2). After completion of the reaction as monitored by TLC acetonitrile added to the reaction mixture, the catalyst easily collected by means of a magnet to be reused in subsequent reactions. The obtained products recrystallized in ethanol and characterized by 1HNMR, IR and 13CNMR.Spectral data for analysis compounds
Conclusions
In conclusion, designing, synthesis, full characterization and application of a novel magnetically separable dendrimeric core of oxo vanadium phthalocyanine were described. The catalytic activity of the catalyst was also studied on the synthesis of 3,4-dihydropyrano[c]chromenes. The heterogenized catalyst can be readily recovered by using an external magnet, which eliminates tedious work-up and catalyst filtration procedures. Recycled catalyst was reused for 8 times without any significant losing its activity. Other merits of the protocol are the use of a chemically stable oxidant, the mild and green reaction conditions, operational simplicity, short reaction times, room temperature of the reaction and solvent free condition. Further research for systematic and knowledge-based development of this field is going on in our research group.Acknowledgements
The authors gratefully acknowledge the Bu-Ali Sina and Nahavand, University Research Councils, and also Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS), for providing support to this work.Notes and references
- N. Azgomi and M. Mokhtary, J. Mol. Catal. A: Chem., 2015, 398, 58–64 CrossRef CAS.
- M. A. Zolfigol, V. Khakyzadeh, A. R. Moosavi-Zare, A. Rostami, A. Zare, N. Iranpoor, M. H. Beyzavie and R. Luque, Green Chem., 2013, 15, 2132–2140 RSC.
- F. Ahangaran, A. Hassanzadehand and S. Nouri, Int. Nano Lett., 2013, 3, 23–28 CrossRef.
- H. Uchida, P. Y. Reddy, S. Nakamura and T. Toru, J. Org. Chem., 2003, 68, 8736–8738 CrossRef CAS PubMed.
- G. Mbambisa and T. Nyokong, Polyhedron, 2008, 27, 2799–2804 CrossRef CAS.
- F. Meng, R. Zhao, Y. Zhan, Y. Lei, J. Zhong and X. Liu, Mater. Lett., 2011, 65, 264–267 CrossRef CAS.
- A. Shaabani, S. Keshipour, M. Hamidzad and S. Shaabani, J. Mol. Catal. A: Chem., 2014, 395, 494–499 CrossRef CAS.
- (a) P. Kivits, R. Debont and J. van der Veen, Appl. Phys. A: Solids Surf., 1981, 26, 101–105 CrossRef; (b) D. Gu, Q. Chen, J. Shu, X. Tang, F. Gan, S. Shen, K. Liu and H. Xu, Thin Solid Films, 1995, 257, 88–93 CrossRef CAS.
- G. Long, B. Snedecker, K. Bartosh, M. L. Werner and A. Sen, Can. J. Chem., 2001, 79, 1026–1029 CrossRef CAS.
- (a) S. Wu, A. Sun, F. Zhai, J. Wang, W. Xu, Q. Zhang and A. A. Volinsky, Mater. Lett., 2011, 65, 1882–1884 CrossRef CAS; (b) K. S. Lee, M. H. Woo, H. S. Kim, E. Y. Lee and I. S. Lee, Chem. Commun., 2009, 3780–3782 RSC.
- K. Niknam and A. Piran, Green Sustainable Chem., 2013, 3, 1–8 CrossRef.
- (a) W. O. Foye, Prinicipi di Chemico Farmaceutica, Piccin, Padova, Italy, 1991, p. 416 Search PubMed; (b) G. R. Green, J. M. Evans and A. K. Vong, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Ress and E. F. V. Scriven, Pergamon Press, Oxford, 1995, vol. 5, p. 469 Search PubMed; (c) Y. L. Zhang, B. Z. Chen, K. Q. Zheng, M. L. Xu and X. H. Lei, Chin. Acta Pharm. Sin., 1982, 17, 17–22 (Chem. Abstr., 1982, 96, 135383e) CAS; (d) L. Bonsignore, G. Loy, D. Secci and A. Calignano, Eur. J. Med. Chem., 1993, 28, 517–520 CrossRef CAS; (e) L. L. Andreani and E. Lapi, Boll. Chim. Farm., 1960, 99, 583–586 Search PubMed; (f) E. C. Witte, P. Neubert and A. Roesch, Ger. Offen., 1986, 3427985 (Chem. Abstr., 1986, 104, 224915f) Search PubMed; (g) R. M. Shaker, Pharmazie, 1996, 51, 148–151 CAS.
- S. Abdolmohammadi and S. Balalaie, Tetrahedron Lett., 2007, 48, 3299–3303 CrossRef CAS.
- M. Kidwai and S. Saxena, Synth. Commun., 2006, 36, 2737–2742 CrossRef CAS.
- M. M. Heravi, B. Alimadadi Jani, F. Derikvand, F. F. Bamoharram and H. A. Oskooie, Catal. Commun., 2008, 10, 272–275 CrossRef CAS.
- M. Seifi and H. Sheibani, Catal. Lett., 2008, 126, 275–279 CrossRef CAS.
- J. M. Khurana and S. Kumar, Tetrahedron Lett., 2009, 50, 4125–4127 CrossRef CAS.
- H. Mehrabi and H. Abusaidi, J. Iran. Chem. Soc., 2010, 7, 890–894 CrossRef CAS.
- J. M. Khurana, B. Nand and P. Saluja, Tetrahedron, 2010, 66, 5637–5641 CrossRef CAS.
- M. M. Heravi, M. Zakeri and N. Mohammadi, Chin. J. Chem., 2011, 29, 1163–1166 CrossRef CAS.
- H. Nagabhushana, S. S. Saundalkar, L. Muralidhar, B. M. Nagabhushana, C. R. Girija, D. Nagaraja, M. A. Pasha and V. P. Jayashankara, Chin. Chem. Lett., 2011, 22, 143–146 CrossRef CAS.
- H. Mehrabi and M. Kazemi-Mireki, Chin. Chem. Lett., 2011, 22, 1419–1422 CrossRef CAS.
- K. Niknam and A. Jamali, Chin. Chem. Lett., 2012, 33, 1840–1849 CAS.
- (a) P. Salehi, M. A. Zolfigol, F. Shirini and M. Baghbanzadeh, Curr. Org. Chem., 2006, 10, 2171–2189 CrossRef CAS; (b) M. Daraei, M. A. Zolfigol, F. Derakhshan-Panah, M. Shiri, H. G. Kruger and M. Mokhlesi, J. Iran. Chem. Soc., 2015, 12, 855–861 CrossRef CAS; (c) D. Azarifar, S. M. Khatami, M. A. Zolfigol and R. Nejat-Yami, J. Iran. Chem. Soc., 2014, 11, 1223–1230 CrossRef CAS; (d) M. Safaiee, M. A. Zolfigol, M. Tavasoli and M. Mokhlesi, J. Iran. Chem. Soc., 2014, 11, 1593–1597 CrossRef CAS.
- F. Shirini, M. A. Zolfigol, P. Salehi and M. Abedini, Curr. Org. Chem., 2008, 12, 183–202 CrossRef CAS.
- (a) M. A. Zolfigol, T. Azadbkht, V. Khakizadeh, R. Nejatyami and D. Perrin, RSC Adv., 2014, 4, 40036–40042 RSC; (b) T. Azadbakht, M. A. Zolfigol, R. Azadbakht, V. Khakizadeh and D. Perrin, New J. Chem., 2015, 39, 439–444 RSC.
- M. A. Zolfigol, A. R. Pourali, S. Sajjadifar and S. Farahmand, Curr. Catal., 2013, 2, 151–158 CrossRef CAS.
- J. Alzeer, P. J. C. Roth and N. W. Luedtke, Chem. Commun., 2009, 1970–1971 RSC.
- G. Mbambisa and T. Nyokong, Polyhedron, 2008, 27, 2799–2804 CrossRef CAS.
- S. Qu, H. Yang, D. Ren, S. Kan, G. Zou, D. Li and M. Li, J. Colloid Interface Sci., 1999, 215, 190–192 CrossRef CAS PubMed.
- P. Modisha, T. Nyokong and E. Antunes, J. Mol. Catal. A: Chem., 2013, 380, 131–138 CrossRef CAS.
- H. Moghanian, A. Mobinikhaledi, A. G. Blackmanc and E. Sarough-Farahanib, RSC Adv., 2014, 4, 28176–28185 RSC.
- M. Kassaee, H. Masrouri and F. Movahed, Appl. Catal., A, 2011, 395, 28–33 CrossRef CAS.
- M. Esmaeilpour, A. R. Sardarian and J. Javidi, Appl. Catal., A, 2012, 445, 359–367 CrossRef.
- M. Khoobia, L. Ma'mania, F. Rezazadehb, Z. Zareieb and A. Foroumadia, J. Mol. Catal. A: Chem., 2012, 359, 74–80 CrossRef.
- (a) R. Ghorbani-Vaghei, Z. Toghraei-Semiromi and R. Karimi-Nami, J. Braz. Chem. Soc., 2011, 22, 905 CAS; (b) J. P. Patel, J. R. Avalani and D. K. Raval, J. Chem. Sci., 2013, 125, 531 CrossRef CAS; (c) A. Montaghami and N. Montazeri, Orient. J. Chem., 2014, 30, 1361 CrossRef CAS; (d) N. Hazeri, M. T. Maghsoodlou, F. Mir, M. Kangani, H. Saravani and E. Molashahi, Chin. J. Catal., 2014, 35, 391 CrossRef; (e) A. Shaabani, S. Samadi, Z. Badri and A. Rahmati, Catal. Lett., 2005, 104, 39 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18723k |
| This journal is © The Royal Society of Chemistry 2015 |