Effect of alkyl groups on emission properties of aggregation induced emission active N-alkyl arylaminomaleimide dyes†
Hiroaki Imotoa,
Katsuya Nohmia,
Kohei Kizakia,
Seiji Wataseb,
Kimihiro Matsukawab,
Shunsuke Yamamotoc,
Masaya Mitsuishic and
Kensuke Naka*a
aFaculty of Molecular Chemistry and Engineering, Graduate School of Science and Technology, Kyoto Institute of Technology, Goshokaido-cho, Matsugasaki, Sakyo-ku, Kyoto 606-8585, Japan. E-mail: kenaka@kit.ac.jp
bOsaka Municipal Technical Research Institute, 1-6-50 Morinomiya, Joto-ku, Osaka 536-8553, Japan
cInstitute of Multidisciplinary Research for Advanced Materials (IMRAM), Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan
First published on 27th October 2015
Abstract
Aggregation induced emission (AIE) active N-alkyl aminomaleimide dyes with various kinds of N-alkyl groups were synthesized in a one pot process. The synthesized dyes exhibited different emission behaviors depending on the chemical structure of the N-alkyl group, although they had no direct contribution to the π-conjugated system. Chain length, hydroxyl group and branching structure were important in the emission properties such as quantum yield and emission wavelength. Furthermore, it was found that one of the dyes showed mechanochromism; the green emission of crystal samples turned to blue emission after grinding. Single crystal X-ray diffraction analysis revealed that the surrounding around the luminophore was dominant in the emission behaviors.
Introduction
Solid state emission is a challenging matter for the development of luminescent materials,1 and a large number of studies have been carried out for intense emission in the solid state.2 Among them, the aggregation induce emission (AIE) system has contributed a great deal to advancing this field.3 Since the first report on AIE in 2001,4 various kinds of AIE active dyes have been developed; tetraphenyl silole,4,5 tetraphenyl ethylene (TPE),6 and so on.7 While no emission is observed in solutions, the AIE active dyes show emission in the solid state. Excitons of these AIE active dyes were deactivated without radiation in solutions due to molecular motions, whereas aggregation formation restricts these deactivation pathways. The state of the samples (e.g. solution, aggregation and solid) drastically changes the emission properties, and the environmentally-responsive property has been widely applied to sensors and bioprobes.8,9 An important factor for solid state emission lies in the fashion of molecular packing, which are highly influenced by the substituents around the luminophores. This is why chemical structure which is not included in the π-conjugated system of the luminescent centers should be considered for molecular design of the AIE active dyes.10 Actually, side chains influence emission properties such as emission color and quantum yield. For example, the emission behavior of TPE was changed by chain length of the alkyl chain around the TPE luminescent center.10cRecently, we have reported AIE active N-aryl arylaminomaleimides (Chart 1a)11 and N-alkyl arylaminomaleimides (Chart 1b).12 The dye skeleton can be constructed in a one-pot process, and the obtained luminescent dyes are attractive because of their simple structures and versatile molecular design. The emission properties of N-aryl aminomaleimides were controlled by substituents on the 2- and 3-positions of the maleimide ring because the π-conjugated system is expanded to these substituents.13,14 In addition, we found that the N-aryl group of the maleimides also affected the emission properties despite the little contribution to the π-conjugated system.13 This is because the molecular packing was changed by the N-aryl groups; the local surroundings around the maleimide ring highly influenced their emission properties. A maleimide ring has two carbonyl groups, which easily form interactions such as hydrogen bond in the solid states. Thus, short contacts to the luminescent center can be potentially controlled in a diverse way, leading to various emission behaviors of the AIE active aminomaleimide dyes.
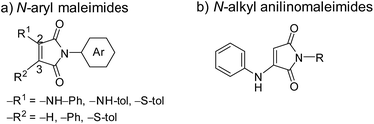 | ||
| Chart 1 Chemical structure of maleimide dyes. (a) N-Aryl maleimides, and (b) N-alkyl anilinomaleimides. | ||
In a previous paper, we reported that N-alkyl aminomaleimides (Chart 1b) have advantages in high quantum yields, easy synthesis and good solubility, in comparison with N-aryl aminomaleimides.12 N-Alkyl groups may work as steric hinderance around the maleimide ring to avoid co-facial π–π stacking toward the intense emission in the solid states. Therefore, it can be expected that the screening of the N-alkyl group realizes a variety of emission properties despite no direct electronic interaction to the π-conjugated system. Herein, we investigated effect of the N-alkyl groups of N-alkyl anilinomaleimides in a systematic way. Alkyl chain length and hydroxyl group and branching structure drastically changed emission properties such as emission wavelength and quantum yield. Furthermore, one of the synthesized dyes showed mechanochromism,15,16 differently from the others. X-ray diffraction study revealed that molecular packing was changed in dependent on the N-alkyl group. This is, to the best of our knowledge, the first study on the relationship between the N-alkyl group and solid state emission of maleimide dyes, though various kinds of luminescent maleimide dyes have been reported to date.11–14
Results and discussion
Synthesis
Synthesis of N-alkyl anilinomaleimides is shown in Scheme 1.17 Dimethyl acetylenedicarboxylate (1) and one equivalent of aniline were reacted at ambient temperature to give an adduct product, dimethyl anilinofumarate (2) nearly quantitatively. Subsequently, unreacted compounds were removed in vacuo. Without further purification, excess amount of a primary alkyl amine in methanol was added to 2 at ambient temperature. After stirring overnight, the reaction mixture was cooled to −20 °C to give N-alkyl anilinomaleimides (3–5) as yellow solids. The isolated yields of some products were relatively low because the obtained N-alkyl anilinomaleimides were slightly soluble in methanol. Notably, the synthetic procedure was one-pot, and the purification process was only recrystallization from a reaction solution.Alkyl chain length was examined from C1 to C12 in 3a–h, and cyclohexyl group was employed in 3i as a relatively rigid substituent. Hydroxyl group was introduced in 4a–c to provide a hydrogen bond donor. For incorporation of branching structure, chiral primary amines were used for (S)-5 and (R)-5, and the racemic mixture rac-5 was also synthesized. The chemical structures of the newly synthesized compounds were determined with 1H and 13C NMR and high resolution mass spectroscopy.
Optical properties in general
The optical properties are summarized in Table 1. The absorption maxima were 380–383 nm in solutions (c = 1.0 × 10−4 mol L−1 in chloroform), suggesting that the N-alkyl groups had no significant contribution to the π-conjugated systems in dilute solutions. Solid samples for photophysical measurement were prepared by reprecipitation in methanol. All of the obtained samples showed luminescence in the solid state, despite no emission in good solvents such as chloroform, dichloromethane, THF and so on. It implies that these dyes are AIE active. Emission wavelength and quantum yield of the dyes were dependent on the N-alkyl groups, suggesting that the surroundings of the aggregated molecules are different from each other.| Dye | λabsa [nm] | λemb [nm] | Φc |
|---|---|---|---|
| a Absorption maxima in CHCl3.b Emission maxima.c Absolute quantum yield in the solid state. | |||
| 3a | 380 | 491 | <0.01 |
| 3b | 380 | 498 | 0.32 |
| 3c | 380 | 497 | 0.56 |
| 3d | 380 | 495 | 0.59 |
| 3e | 380 | 492 | 0.57 |
| 3f | 380 | 492 | 0.48 |
| 3g | 380 | 495 | 0.41 |
| 3h | 380 | 494 | 0.44 |
| 3i | 380 | 492 | 0.45 |
| 4a | 382 | 516 | 0.18 |
| 4b | 383 | 494 | 0.17 |
| 4c | 381 | 496 | 0.11 |
| rac-5 | 382 | 508 | 0.02 |
| (R)- and (S)-5 | 382 | 507 | 0.02 |
Effect of chain length
Methyl (3a), ethyl (3b), propyl (3c), butyl (3d), pentyl (3e), hexyl (3f), octyl (3g), dodecyl (3h) and cyclohexyl (3i) groups were examined to investigate chain length effect (Fig. 1). The emission wavelengths of 3a–i were approximately the same (491–497 nm), while quantum yields were completely different. Although the emissions of 3b–h were intense (Φ = 0.32–0.59), that of 3a was too weak to observe by naked eyes. This is probably because sterically small alkyl groups such as methyl group cannot prevent a luminescent center from concentration self-quenching. For the same reason, 3b (Φ = 0.32) showed weaker emission than 3c–e (Φ > 0.5). Therefore, a certain level of steric hinderance of the N-alkyl chain is necessary for intense solid state emission of the N-alkyl aminomaleimide dyes. | ||
| Fig. 1 (a) Photographs of 3a–h under UV irradiation at 365 nm. (b) Absolute quantum yields of 3a–3h in the solid state. | ||
On the other hand, 3f–i had relatively low quantum yields in comparison with 3c–e, suggesting that the elongation of the alkyl chain offers flexibility and space which enable molecular motions. As a result, butyl group possessed moderate balance between steric hindrance and molecular rigidity. Judging from the results of 3f and 3i, there was no significant difference between hexyl and cyclohexyl groups, and topological effect was not observed.
The lifetimes of 3a–h were measured to examine the components of their emission in the solid state. The dyes had two distinct lifetimes (Fig. S23 and Table S3†). The ratio of shorter and longer lifetime components of 3a (τ1 = 1.11 ns (84%), τ2 = 4.57 ns (16%)) was different from those of 3b–h; elongation of the N-alkyl chain from methyl group raised the ratio of the longer lifetime component (approximately 30%), which is relatively easily deactivated. This means that small substituents such as methyl group cannot sufficiently prevent the excitons from non-radiative deactivation. Despite the minor difference, the emissions of 3b–h were derived from the similar fluorophore, suggesting that the N-alkyl chain did not directly influence the luminescent center.
Effect of hydroxyl group
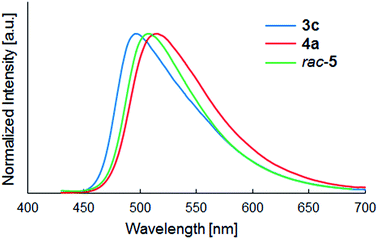
In the case of 4 and 5, the hydroxyl group was incorporated into the N-alkyl chain. Although the UV-vis absorption spectra of all dyes 3–5 were approximately same, the introduction of the hydroxyl group changed emission properties in the solid state. The quantum yields of 5 were low (Φ = 0.02) in comparison with those of 4 (Φ = 0.11–0.18). Branching structure could change the packing structure to cause the significant decrease of quantum yield, though the detailed structural difference was unclear. The circular dichromism (CD) and circularly polarized luminescence (CPL) spectra of (S)- and (R)-5 were silent because the small methyl group of N-alkyl chain has no structural influence on the π-conjugated system in solutions.18Notably, the emission maxima of 4a (λmax = 516 nm) and 5 (λmax = 507–508 nm) were red-shifted in comparison with those of 3 (λmax = 491–498 nm). As representative examples, the PL spectra of 3c, 4a and rac-5 are shown in Fig. 2. It is possible that intermolecular and/or intramolecular interaction, e.g. hydrogen bond, between the hydroxyl group and the maleimide moiety caused lowering the LUMO level in the solid states. On the other hand, no significant red-shift of emission was observed in the case of 4b and 4c, which possessed longer alkyl chain than 4a.
For structural analysis in the solid state, the IR spectra of 3c, 4a–c and rac-5 were measured with KBr method (Fig. 3). The signals of the carbonyl group of 4a (1650 cm−1) and rac-5 (1644 cm−1) were shifted from those of the others (1632–1637 cm−1), suggesting the formation of interactions such as hydrogen bonds. In addition, the signals of the hydroxyl groups were defined in the case of 4a (3393 cm−1) and rac-5 (3500 cm−1). This is because 4a and rac-5 formed rigid structure based on the defined interaction, while 4b and 4c adopted various conformation in the solid state due to their longer alkyl chains. These results support the phenomenon in which only 4a and rac-5 exhibited different emission color. In the previous paper, we reported that the LUMO level is stabilized as the number of hydrogen bonds increase, resulting in red-shift of the emission.13 The hydroxyl groups probably directly or indirectly influenced the electronic surroundings of the maleimide luminescent center to cause the emission color change.
Mechanochromism
In the course of the investigation, we found that propyl-substitued dye 3c displayed mechanochromism (Fig. 4). To demonstrate the mechanochromic behavior, crystal samples of 3c (3ccry) were prepared by recrystallization from dichloromethane and methanol, and 3ccry was ground in a mortar to obtain 3cgrd. The emission color of 3ccry was green (502 nm),19 while that of 3cgrd was greenish blue (489 nm). | ||
| Fig. 4 (a) Photographs (excited at 365 nm) and (b) PL spectra of 3ccry and 3cgrd (excited at 350 nm). | ||
For understanding on this phenomenon, single crystal X-ray diffraction (XRD) was carried out. As a representative dye, which did not show mechanochromism, 3i was selected. Suitable single crystals of 3c and 3i were prepared for the measurement. The molecules of 3c formed double hydrogen bonds (2.2 Å) between the secondary amine and carbonyl group (Fig. 5). In addition, short contacts between the protons of the benzene and maleimide rings and the carbonyl group were also observed (2.5–2.7 Å), and the weak hydrogen bonds constructed one-dimensional structure. A mechanical stimulus broke a part of these hydrogen bonds, and the surroundings of the luminophore were altered, resulting in the emission color change. The powder XRD pattern of 3c was simulated based on single crystal XRD. After grinding crystals of 3c, the broad signals were observed (Fig. S25†), suggesting the crystal structure was partial collapsed by grinding.
 | ||
| Fig. 5 Results of single crystal X-ray diffraction analysis on 3c. (a) Packing structure, (b) hydrogen bonds. | ||
On the other hand, the result of 3i showed the molecules formed hydrogen bonds (2.1 Å) between the secondary amine and carbonyl group in a one-dimensional polymer fashion (Fig. 6). Three-dimensional structure was supported by the short contacts (2.5 Å and 2.7 Å) via the hydrogen bonds between the protons of the cyclohexyl groups and the carbonyl groups. The robust network structure could not be collapsed by a mechanical stimulus. It is possible that the dyes other than 3c adopted packing structures similar to that of 3i, resulting in the absence of mechanochromic property.
Conclusions
We have systematically investigated effect of N-alkyl groups on the emission properties of AIE active aminomaleimide dyes. The UV-vis absorption and CD spectra suggested that the N-alkyl groups had no significant direct contribution to the π-conjugated system of the luminophore. Nevertheless, the chain length, hydroxyl group and branching structure were very important in the solid state emission. This is because the N-alkyl groups determined the packing structure, which was dominant in the surroundings of the luminophore. Interestingly, only 3c showed mechanochromism. Single crystal X-ray diffraction analysis revealed that the packing structure of 3c was completely different from that of 3i. A mechanical stimulus may cleave the hydrogen bonds to cause blue shift. This is the first study on the effect of the N-alkyl group of maleimide dyes on the emission properties, to the best of our knowledge. Easy synthesis and tunable emission properties are advantageous in practical use of the AIE active dyes. Further modification and functionalization of maleimide dyes are under way.Experimental
Material
Methanol and dichloromethane were purchased from Nacalai Tesque, Inc. Dimethyl acetylenedicarboxylate was purchased from Tokyo Chemical Industry Co., Ltd. Aniline was purchased from Wako Pure Chemical Industry, Ltd. 3c, 3f and 3g were prepared by following the literature.12Material
1H (400 MHz) and 13C (100 MHz) NMR spectra were recorded on a Bruker DPX-400 spectrometer, and samples were analyzed in CDCl3 using Me4Si as an internal standard. The following abbreviations are used; s: singlet, d: doublet, m: multiplet. High-resolution mass spectra (HRMS) were obtained on a JEOL JMS-SX102A spectrometer. The UV-vis spectra were recorded on a Jasco spectrophotometer V-670 KKN. Emission spectra were obtained on a JASCO fluorescence spectrophotometer FP-8500, and absolute PL quantum yields (Φ) were determined using a JASCO ILFC-847S. The CD and UV-vis spectra were recorded on a JASCO J-820 spectropolarimeter with THF as a solvent at room temperature.X-ray crystallographic data for single crystalline products
The single crystal was mounted on glass fibers with epoxy resin. Intensity data were collected at room temperature on a Rigaku RAXIS RAPID II imaging plate area detector with graphite monochromated Mo Kα radiation. The crystal-to-detector distance was 127.40 mm. Readout was performed in the 0.100 mm pixel mode. The data were collected at room temperature to a maximum 2θ value of 55.0°. Data were processed by the PROCESS-AUTO20 program package. An empirical or numerical absorption correction21 was applied. The data were corrected for Lorentz and polarization effects. A correction for secondary extinction22 was applied. The structure was solved by heavy atom Patterson methods23 and expanded using Fourier techniques.24 Some non-hydrogen atoms were refined anisotropically, while the rest were refined isotropically. Hydrogen atoms were refined using the riding model. The final cycle of full-matrix least-squares refinement on F2 was based on observed reflections and variable parameters. In the case of the crystalline product recrystallized from acetone, the final cycle of full-matrix least-squares refinement on F was based on observed reflections and variable parameters. All calculations were performed using the crystal structure25,26 crystallographic software package. Crystal data and more information on X-ray data collection are summarized in Table S1 and S2.†General synthetic procedure
A dichloromethane solution of 1 (1.51 g, 10.7 mmol) was cooled to 0 °C with ice-water bath, and aniline (1.00 g, 10.7 mmol) was added dropwise. The reaction mixture was stirred at ambient temperature, and turned yellow gradually. After stirring overnight, the volatiles were removed in vacuo to obtain 2 as yellow oil product. Without further purification, a methanol solution (5 mL) of 2 was prepared. To the solution was added primary amine (32.1 mmol, 3 equivalent). After stirring overnight at ambient temperature, the reaction mixture was cooled to −20 °C to obtain N-alkyl aminomaleimide as yellow solid. Unless solid products were obtained, the volatiles were removed in vacuo, and small amounts of methanol (<5 mL) were added. Subsequently, the solution was cooled to −20 °C to isolate N-alkyl aminomaleimide.Acknowledgements
We thank Prof. Y. Chujo and Prof. Y. Morisaki of Kyoto University for measurement of CD spectra. This study is a part of a Grant-in-Aid for Scientific Research on Innovative Areas “New Polymeric Materials Based on Element-Blocks (No. 2401)” (No. 24102003) of The Ministry of Education, Culture, Sports, Science and Technology, Japan.Notes and references
- (a) K.-T. Wong, Y.-Y. Chien, R.-T. Chen, C.-F. Wang, Y.-T. Lin, H.-H. Chiang, P.-Y. Hsieh, C.-C. Wu, C. H. Chou, Y. O. Su, G.-H. Lee and S.-M. Peng, J. Am. Chem. Soc., 2002, 124, 11576 CrossRef CAS PubMed; (b) Z. Xie, B. Yang, F. Li, G. Cheng, L. Liu, G. Yang, H. Xu, L. Ye, M. Hanif, S. Liu, D. Ma and Y. Ma, J. Am. Chem. Soc., 2005, 127, 14152 CrossRef CAS PubMed; (c) C.-H. Zhao, A. Wakamiya, Y. Inukai and S. Yamaguchi, J. Am. Chem. Soc., 2006, 128, 15934 CrossRef CAS PubMed; (d) A. Wakamiya, K. Mori and S. Yamaguchi, Angew. Chem., Int. Ed., 2007, 46, 4273 CrossRef CAS PubMed; (e) C.-H. Zhao, A. Wakamiya and S. Yamaguchi, Macromolecules, 2007, 40, 3898 CrossRef CAS; (f) M. Shimizu, K. Mochida and T. Hiyama, Angew. Chem., Int. Ed., 2008, 47, 9760 CrossRef CAS PubMed; (g) W.-Y. Lai, R. Xia, Q.-Y. He, P. A. Levermore, W. Huang and D. D. C. Bradley, Adv. Mater., 2009, 21, 355 CrossRef CAS.
- Recent papers, see: (a) R. Yoshii, A. Hirose, K. Tanaka and Y. Chujo, J. Am. Chem. Soc., 2014, 136, 18131 CrossRef CAS PubMed; (b) R. Yoshii, A. Hirose, K. Tanaka and Y. Chujo, Chem.–Eur. J., 2014, 20, 8320 CrossRef CAS PubMed; (c) P. Galer, R. C. Korošec, M. Vidmar and B. Šket, J. Am. Chem. Soc., 2014, 136, 7383 CrossRef CAS PubMed; (d) M. V. R. Raju and H.-C. Lin, Org. Lett., 2014, 16, 5564 CrossRef PubMed; (e) A. Goel, A. Sharma, M. Rawat, R. S. Anand and R. Kant, J. Org. Chem., 2014, 79, 10873 CrossRef CAS PubMed; (f) H. Braunschweig, S. Ghosh, J. O. C. Jimenez-Halla, J. H. Klein, C. Lambert, K. Radacki, A. Steffen, A. Vargas and J. Wahler, Chem.–Eur. J., 2015, 21, 210 CrossRef CAS PubMed.
- Reviews, see: (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332 RSC; (b) D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441 CrossRef CAS PubMed; (c) R. Hu, N. L. C. Leung and B. Z. Tang, Chem. Soc. Rev., 2014, 43, 4494 RSC; (d) J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740 RSC.
- Z. Li, Y. W. Dong, J. W. Y. Lam, J. Sun, A. Qin, M. Haüßler, M. Y. P. Dong, H. H. Y. Sung, I. D. Williams, H. S. Kwok and B. Z. Tang, Adv. Funct. Mater., 2009, 19, 905 CrossRef CAS.
- (a) W. Z. Yuan, S. Chen, J. W. Y. Lam, C. Deng, P. Lu, H. H.-Y. Sung, I. D. Williams, H. S. Kwok, Y. Zhang and B. Z. Tang, Chem. Commun., 2011, 47, 11216 RSC; (b) Y. Liu, S. Chen, J. W. Y. Lam, P. Lu, R. T. K. Kwok, F. Mahtab, H. S. Kwok and B. Z. Tang, Chem. Mater., 2011, 23, 2536 CrossRef CAS; (c) J. Huang, X. Yang, J. Wang, C. Zhong, L. Wang, J. Qin and Z. Li, J. Mater. Chem., 2012, 22, 2478 RSC.
- (a) S. Kamino, Y. Horio, S. Komeda, K. Minoura, H. Ichikawa, J. Horigome, A. Tatsumi, S. Kaji, T. Yamaguchi, Y. Usami, S. Hirota, S. Enomoto and Y. Fujita, Chem. Commun., 2010, 46, 9013 RSC; (b) M. Nakamura, T. Sanji and M. Tanaka, Chem.–Eur. J., 2011, 17, 5344 CrossRef CAS PubMed; (c) T. Hirose, K. Higashiguchi and K. Matsuda, Chem.–Asian J., 2011, 6, 1057 CrossRef CAS PubMed; (d) K. Kokado and Y. Chujo, J. Org. Chem., 2011, 76, 316 CrossRef CAS PubMed; (e) R. Yoshii, A. Nagai, K. Tanaka and Y. Chujo, Chem.–Eur. J., 2013, 19, 4506 CrossRef CAS PubMed.
- Reviews, see: (a) D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441 CrossRef CAS PubMed; (b) X. Zhang, X. Zhang, L. Tao, Z. Chi, J. Xub and Y. We, J. Mater. Chem. B, 2014, 2, 4398 RSC; (c) J. Liang, B. Z. Tang and B. Liu, Chem. Soc. Rev., 2015, 44, 2798 RSC.
- Recent papers, see: (a) W. Zhang, R. T. K. Kwok, Y. Chen, S. Chen, E. Zhao, C. Y. Y. Yu, J. W. Y. Lam, Q. Zheng and B. Z. Tang, Chem. Commun., 2015, 51, 9022 RSC; (b) Y. Zhao, C. Y. Y. Yu, R. T. K. Kwok, Y. Chen, S. Chen, J. W. Y. Lam and B. Z. Tang, J. Mater. Chem. B, 2015, 3, 4993 RSC; (c) D. Yoshihara, T. Noguchi, Y. Tsuchiya, B. Roy, T. Yamamoto and S. Shinkai, Chem. Lett., 2015, 44, 812 CrossRef CAS; (d) R. Zhang, R. T. K. Kwok, B. Z. Tang and B. Liu, RSC Adv., 2015, 5, 28332 RSC; (e) H. Arora, V. Bhalla and M. Kumar, RSC Adv., 2015, 5, 32637 RSC; (f) L. Ji, Y. Guo, S. Hong, Z. Wang, K. Wang, X. Chen, J. Zhang, J. Hu and R. Pei, RSC Adv., 2015, 5, 36582 RSC.
- (a) P. An, Z.-F. Shi, W. Dou, X.-P. Cao and H.-L. Zhang, Org. Lett., 2010, 12, 4364 CrossRef CAS PubMed; (b) X. Zhang, Z. Ma, Y. Yang, X. Zhang, Z. Chi, S. Liu, J. Xu, X. Jia and Y. Wei, Tetrahedron, 2014, 70, 924 CrossRef CAS; (c) Y. Dong, W. Wang, C. Zhong, J. Shi, B. Tong, X. Feng, J. Zhi and Y. Dong, Tetrahedron Lett., 2014, 55, 1496 CrossRef CAS.
- T. Kato and K. Naka, Chem. Lett., 2012, 41, 1445 CrossRef CAS.
- K. Kizaki, H. Imoto, T. Kato and K. Naka, Tetrahedron, 2015, 71, 643 CrossRef CAS.
- H. Imoto, K. Kizaki, S. Watase, K. Matsukawa and K. Naka, Chem.–Eur. J., 2015, 21, 12105 CrossRef CAS PubMed.
- (a) H.-C. Yeh, W.-C. Wu and C.-T. Chen, Chem. Commun., 2003, 404 RSC; (b) K. Onimura, M. Matsushima, K. Yamabuki and T. Oishi, Polym. J., 2010, 42, 290 CrossRef CAS; (c) M. Nakamura, K. Yamabuki, T. Oishi and K. Onimura, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4945 CrossRef CAS.
- Reviews, see: (a) Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878 RSC; (b) X. Zhang, Z. Chi, Y. Zhang, S. Liu and J. Xu, J. Mater. Chem. C, 2013, 1, 3376 RSC.
- On mechanochromic maleimide dyes, see: (a) J. Luo, L.-Y. Li, Y. Song and J. Pei, Chem.–Eur. J., 2011, 17, 10515 CrossRef CAS PubMed; (b) H. Imoto, K. Kizaki and K. Naka, Chem.–Asian J., 2015, 10, 1698 CrossRef CAS PubMed.
- (a) N. D. Heindel, V. B. Fish and T. F. Lemke, J. Org. Chem., 1968, 33, 3997 CrossRef CAS; (b) N. D. Heindel, J. Org. Chem., 1970, 35, 3138 CrossRef CAS.
- The samples for CD and CPL measurements were solutions (c = 1.0 × 10−5 mol L−1 in THF) and dispersions (THF/water (v/v) = 1/99), respectively. For CD spectra, see ESI†.
- The emission wavelength of 3ccry (λ = 502 nm) was slightly different from that of the samples obtained from reprecipitation (3c in Table 1, λ = 497 nm). The samples from reprecipitation may include a small amount of amorphous domains.
- PROCESS-AUTO, RAXIS data-processing software, Rigaku Corporation, Tokyo, Japan, 1996 Search PubMed.
- T. Higashi, FS_ABSCOR, Program for Absorption Correction, Rigaku Corporation, Tokyo, Japan, 1995 Search PubMed.
- A. C. Larson, Crystallographic Computing, ed. F. R. Ahmed, Munksgaard, Copenhagen, Denmark, 1970, pp. 291−294 Search PubMed.
- P. T. Beurskens, G. Admiraal, G. Beurskens, W. P. Bosman, R. de Gelder, R. Israel and J. M. M. Smits, The DIRDIF-99 Program System, Technical Report of the Crystallography Laboratory, University of Nijmegen, Nijmegen, The Netherlands, 1999 Search PubMed.
- P. T. Beurskens, G. Admiraal, G. Beurskens, W. P. Bosman, S. Garcia-Granda, R. O. Gould, J. M. M. Smits and C. Smykalla, PATTY, The DIRDIF Program System, Technical Report of the Crystallography Laboratory, University of Nijmegen, Nijmegen, The Netherlands, 1992 Search PubMed.
- CrystalStructure 3.8: Crystal Structure Analysis Package, Rigaku Americas, The Woodlands, TX, 2000 Search PubMed.
- J. R. Carruthers, J. S. Rollett, P. W. Betteridge, D. Kinna, L. Pearce, A. Larsen and E. Gabe, CRYSTALS Issue 11; Chemical Crystallography Laboratory, Oxford, U.K., 1999 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectrum data. CCDC 1423627 and 1423628. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra18690k |
| This journal is © The Royal Society of Chemistry 2015 |