A new microporous layer material to improve the performance and durability of polymer electrolyte membrane fuel cells
Abstract
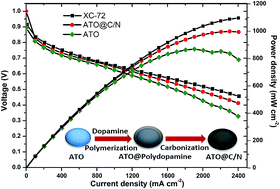
Antimony doped tin oxide (ATO), a kind of semiconducting nanocrystalline material, has excellent electrochemical stability but poor electrical conductivity. Herein, ATO nanocomposites with carbon coatings are prepared by immersing ATO nano-material into dopamine solution, and then thermal treatment to improve the electrical conductivity of the ATO material. The morphology and microstructure of ATO@C/N nanocomposites are characterized using a scanning electron microscope and transmission electron microscopy. The GDLs with the MPL prepared from ATO@C/N nanocomposites are characterized by through-plane resistance testing, mercury intrusion porosimetry and surface contact angle measurement. The results of the above show that ATO@C/N nanocomposites with a 2.16 nm thick carbon coating enhance the electrical conductivity of ATO nanocrystals and exhibit higher electrochemical stability. Further, the performance of MEA fabricated with ATO@C/N as the cathode MPL is evaluated. The maximum power density approaches 1000 mW cm−2, and a slight difference in cell performance is observed compared to XC-72.

 Please wait while we load your content...
Please wait while we load your content...