Eu doped Si-oxynitride fluorescent nanofibrous inorganic membranes with high flexibility†
Abstract
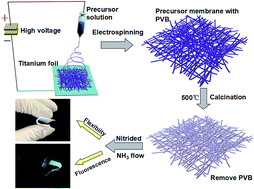
Pure inorganic materials are expected to find applications in various fields including energy-efficient and environmental-friendly soft display technologies, thereby requiring them to operate effectively while being bent or stretched. In the present study, excellent mechanical flexibility properties were successfully imparted into conventionally fragile inorganic materials using a nanobelt network design. Specifically, organic–inorganic composite materials were engineered into considerably long and continuous nanobelts using a simple electrospinning process. The composites were then subjected to calcination and nitridation processes to produce highly fluorescent inorganic (Si-based) membranes with high flexibility. The synthesized nanobelts exhibit high aspect ratios and well-defined rectangular cross-sections, and display excellent mechanical flexibility. The nanobelts could be bent to a minimum curvature radius down to 1 mm. Furthermore, the nanobelts incur no visible damage after 500 cycles of bending to a radius of 1 cm. Under an applied strain of 6% for 500 cycles, the flexible SiO2-based fluorescent nanobelt membrane maintained a high strength of 6.0 MPa. Moreover, the photoluminescence intensity of the free-standing fluorescent nanofibrous inorganic membranes featured excellent environmental, thermal and mechanical cyclic stability. The current findings strongly indicate the great potential of the engineered fluorescent inorganic membranes as fluorescent materials in flexible display technologies and the remote packaging of LED.

 Please wait while we load your content...
Please wait while we load your content...