A novel heterobimetallic Ru(ii)–Gd(iii) complex-based magnetoluminescent agent for MR and luminescence imaging†
Abstract
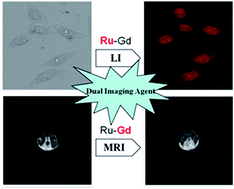
A novel heterobimetallic ruthenium(II)–gadolinium(III) complex, Ru–Gd, comprising a luminescent Ru(II) complex, [Ru(bpy)2(phen)]2+ (bpy: 2,2′-bipyridine; phen: 1,10-phenanthroline), and a Gd(III) complex, DOTA–Gd3+ (DOTA: 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid), has been designed and synthesized as a magnetoluminescent dual-modal imaging agent. The heterobimetallic complex Ru–Gd is water-soluble, biocompatible with low cytotoxicity, strongly luminescent with a long luminescence lifetime and a large Stokes shift, and has comparable longitudinal relaxivity r1 (4.71 mM−1 s−1), which enable the complex to be suitable for luminescence bioimaging and T1-weighted MR imaging. Using Ru–Gd as a contrast agent, the T1-weighted MRI of a Kunming (KM) mouse was successfully carried out. The results demonstrated the availability and validity of our approach for the development of magnetoluminescent dual-modal imaging agents.
- This article is part of the themed collection: RSC Advances Editors' collection: f Block Chemistry

 Please wait while we load your content...
Please wait while we load your content...