Drug-loaded emulsion electrospun nanofibers: characterization, drug release and in vitro biocompatibility
Jue Huab,
Molamma P. Prabhakaran*b,
Lingling Tianb,
Xin Ding*a and
Seeram Ramakrishnab
aCollege of Textiles, Donghua University, Shanghai 201620, China. E-mail: xding@dhu.edu.cn
bSTART-Thrust 3, National University of Singapore, Create Research Wing, #03-08, 1 Create Way, Singapore 138602. E-mail: nanotechmpp@gmail.com
First published on 17th November 2015
Abstract
Emulsion electrospinning is a flexible and promising technique for encapsulating various drugs into nanofibers. In this work, nanofibrous scaffolds were produced by emulsion electrospinning of either metformin hydrochloride (MH) or metoprolol tartrate (MPT) with poly(ε-caprolactone) (PCL) or poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) (PHBV). The influence of preparation processes and emulsion compositions (polymer/drug/surfactant Span 80) towards the drug release behaviour of the scaffolds, together with their morphology, surface and thermal properties were evaluated. In vitro release studies indicated that the emulsion electrospun nanofibers significantly alleviated the burst release and produced a sustained release of drugs compared to the blended electrospun nanofibers. Between the two polymers studied, PCL demonstrated a better drug delivery carrier compared to PHBV, and MPT incorporated nanofibers showed less burst release than the others. The emulsion electrospun nanofibers were evaluated for their cytotoxicity using human mesenchymal stem cells and the cytotoxicity results showed that the emulsion electrospun MPT/PCL scaffold favoured cell growth compared to other tested scaffolds. Our study shows that emulsion electrospinning could be a better technique than normal blend electrospinning, especially in modulating the drug release properties by regulating the oil phase and water phase of the emulsions to obtain the desired drug release for the drug delivery systems. And PCL may be a better drug delivery carrier than PHBV.
1. Introduction
In clinical practice, patients suffering from cardiovascular disease, diabetes, hypertension and other chronic diseases take drugs either orally or by systematic injection. Drugs can be delivered to the target site, but the amount of delivered drug decreases against the initial drug dose because drugs also spread to healthy sites through the blood circulatory system. Due to this reason, patients end up with an excessive intake of drugs, inducing undesirable side effects.1 It is desirable to minimize the use of drugs, while controlled drug release systems help to improve the effectiveness of drugs, reducing the frequency of drug administration and toxicity, enhancing compliance of patients.1 Today, there are numerous controlled release systems, among which the spray-drying and solvent-evaporation techniques have been well studied due to their simple procedures and the potential for industrial scale-up. However, these methods make the leakage of hydrophilic drugs unavoidable and is an obstacle in achieving high encapsulation efficiency. Furthermore, the resultant products obtained by these two techniques are in the powder form, and they cannot directly serve as scaffolds for tissue engineering (TE) unless incorporated into a polymer matrix or sintered together.2 On the other hand, polymeric drug delivery systems (for example, electrospun polymer nanofiber mats) are capable of serving as both drug encapsulation matrix and tissue engineered scaffolds.3–6 Electrospinning is a remarkably simple and powerful technique utilized for the generation of polymeric fibers in the sub-micrometer scale, ranging from about 50 nm to several microns.7 Traditional electrospun nanofibrous drug delivery systems are usually fabricated via blend electrospinning, i.e., by simply mixing drug/protein with the polymer solution. The major disadvantages of the blend-electrospinning procedure include clearance and denaturation of the drug/protein molecules along with their severe burst release phenomenon, so that reducing the effective lifetime of the device, especially during the encapsulation of hydrophilic drugs.8Emulsion electrospinning is a novel process that make use of an emulsion to prepare core–shell nanofibers,7,9–11 and it has been successfully applied for the encapsulation of drugs, proteins and growth factors into the inner core of the nanofibers.12–16 During the emulsification process, hydrophilic drug is dissolved in water (water phase), while hydrophobic polymer is dissolved in the solvent (oil phase). As the oil phase evaporates quickly during the stretching and solidification process, most of the hydrophilic drug gets encapsulated into the fibers instead of escaping onto the fiber surfaces,17 and thus drug burst release can be alleviated or even avoided. Another advantage of emulsion electrospinning is that it can produce nanofibers from dilute polymer solution in good quality,9 making it possible for the commercialization and large-scale production of drug loaded nanofibers. Although different drugs/proteins have been encapsulated within various polymers by emulsion electrospinning, there is a dearth of studies on controlled drug release from emulsion electrospun nanofibers.
As discussed in our former research, the emulsion consists of three important parameters, including surfactants/emulsifiers, oil phase, and water phase.9 The properties of the emulsion electrospun nanofibers are essentially modulated by these three parameters. Therefore, we speculated that a controlled drug release can be gained through manipulation of these three important parameters of emulsion. Two polymers were used in this study, namely poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) or PHBV and poly(ε-caprolactone) or PCL. PHBV is a semi-crystalline polymer, produced by a variety of microorganisms, and it degrades through surface erosion, fact that turns it attractive for controlled drug release applications.18 However studies on the drug release behaviour from PHBV nanofibers are rarely available in the literature. PCL also is a semi-crystalline polymer, and it is frequently used for drug release and tissue regeneration because, besides being biodegradable, its decomposition products are non-toxic and are easily excreted from human organism.19
Chronic cardiovascular diseases, including high blood pressure, blood glucose and cholesterol, afflicts millions of people in the world. Metformin hydrochloride (MH) and metoprolol tartrate (MPT) are two drugs used for the treatment of chronic cardiovascular diseases.20 However oral use of these two hydrophilic drugs is limited by the low systemic bioavailability and short half-life period. There is an urgent need to develop controlled drug delivery system that facilitate the delivery of such drugs in a controlled and systematic fashion. The properties of the drugs also shows that both MH and MPT are freely soluble in water, though MH is more hydrophilic than MPT,20 and at the same time the molecular weight of MH is much smaller than MPT.
Mammalian heart has been considered terminally differentiated with a static number of cardiomyocytes that are incapable of self-renewal.21 Stem cells are nonspecialized cells, which have the potential to create other types of specific cells, such as blood, brain, tissue or muscle-cells. In addition, stem cell therapy shows great potential for regeneration of damaged myocardial tissue. Mesenchymal stem cells (MSCs), the major stem cells for cell therapy, have the potential to differentiate into various types of cells, the ability to self-renew, and possess immunomodulatory properties,22 and MSCs have afforded promise in the treatment of numerous diseases, mainly tissue injury and immune disorders.23 MSCs have also been considered as one of the most promising candidates for biomaterial based cell therapy of cardiac diseases. Therefore, we chose MSCs as the model cells to test the biocompatibility of the cardiac drug encapsulated emulsion electrospun nanofibrous scaffolds.
In this study, we investigated the feasibility of encapsulating either metformin hydrochloride or metoprolol tartrate into PCL or PHBV nanofibers via emulsion electrospinning technique. For comparative purposes, the drug was blended with the respective polymers and electrospun by the process of blend electrospinning. The influence of nanofiber preparation processes and emulsion compositions (polymer/drug/emulsifier Span 80) on the in vitro release profiles of MH and MPT from the nanofibers were studied to evaluate the efficacy of using emulsion electrospun core–shell nanofibers for the sustained release of drugs, and to understand if controlled release can be achieved via regulating the oil phase (polymer) and water phase (drug) of the emulsions.
2. Materials and methods
2.1 Materials
Poly(ε-caprolactone) (Mw 80 kDa), poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) (natural origin, HV content 8 mol%, Mw 400–600 kg mol−1), metformin hydrochloride (MH), metoprolol tartrate salt (MPT), sorbitan monooleate (Span 80), hexamethyldisilazane (HMDS), glutaraldehyde were purchased from Sigma, Singapore. Chloroform (CHCl3) and methanol (MeOH) were purchased from Fisher Scientific Company, UK. Human MSCs were obtained from Lonza, USA. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and trypsin/EDTA were purchased from GIBCO Invitrogen, USA.2.2 Preparation of solutions and electrospinning
Table 1 listed different formulations for preparation of various nanofibers. The ratio of drug and polymer used in drug–polymer nanofibers was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. Selection of this drug
50. Selection of this drug![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) polymer ratio was also based on previous work published by Xu et al.12 and Li et al.24 The drug–polymer emulsion electrospun nanofibers were prepared by individually dissolving PCL or PHBV together with small amounts of surfactants in chloroform, followed by the addition of the aqueous drug solution. Briefly, 400 mg PCL or 650 mg PHBV with appropriate amount of surfactant Span 80 were dissolved in 5 mL chloroform for preparing the oil phase, in which 8% PCL (w/v) with 1% Span 80 (v/v) or 13% PHBV (w/v) with 1% Span 80 (v/v) contained. 2 wt% MH or MPT was dissolved in distilled water and added dropwise to the above solution. All emulsions were stirred for about 2 hours to allow drug to load into the polymer shell and then used immediately for electrospinning. For comparison purposes, PCL and PHBV nanofibers with drug incorporated were also prepared by blend electrospinning, and a mixture solvent of chloroform and methanol (80
polymer ratio was also based on previous work published by Xu et al.12 and Li et al.24 The drug–polymer emulsion electrospun nanofibers were prepared by individually dissolving PCL or PHBV together with small amounts of surfactants in chloroform, followed by the addition of the aqueous drug solution. Briefly, 400 mg PCL or 650 mg PHBV with appropriate amount of surfactant Span 80 were dissolved in 5 mL chloroform for preparing the oil phase, in which 8% PCL (w/v) with 1% Span 80 (v/v) or 13% PHBV (w/v) with 1% Span 80 (v/v) contained. 2 wt% MH or MPT was dissolved in distilled water and added dropwise to the above solution. All emulsions were stirred for about 2 hours to allow drug to load into the polymer shell and then used immediately for electrospinning. For comparison purposes, PCL and PHBV nanofibers with drug incorporated were also prepared by blend electrospinning, and a mixture solvent of chloroform and methanol (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) was used. The amount of drug incorporated for blend electrospinning was the same as those used for emulsion electrospinning. Pure PCL and PHBV were dissolved in chloroform/methanol (80
20) was used. The amount of drug incorporated for blend electrospinning was the same as those used for emulsion electrospinning. Pure PCL and PHBV were dissolved in chloroform/methanol (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) solvent for electrospun and served as the control nanofibers with a concentration of 8% (w/v) and 15% (w/v) respectively.
20) solvent for electrospun and served as the control nanofibers with a concentration of 8% (w/v) and 15% (w/v) respectively.
| Polymer | Scaffolds | Solvents (v/v) | Diameter (nm) | Contact angle (°) |
|---|---|---|---|---|
| a E & B are the abbreviation of emulsion and blend electrospinning, respectively. | ||||
| PCL | Pure PCL | CHCl3/MeOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
352 ± 173 | 136.47 ± 0.84 |
| MH/PCL(E) | CHCl3 | 235 ± 59 | 0 | |
| MH/PCL(B) | CHCl3/MeOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
276 ± 77 | 114.70 ± 2.56 | |
| MPT/PCL(E) | CHCl3 | 306 ± 79 | 0 | |
| MPT/PCL(B) | CHCl3/MeOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
319 ± 83 | 127.83 ± 1.04 | |
| PHBV | Pure PHBV | CHCl3/MeOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
589 ± 198 | 124.03 ± 0.15 |
| MH/PHBV(E) | CHCl3 | 363 ± 92 | 38.10 ± 0.78 | |
| MH/PHBV(B) | CHCl3/MeOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
375 ± 131 | 100.70 ± 0.87 | |
| MPT/PHBV(E) | CHCl3 | 510 ± 143 | 39.20 ± 2.96 | |
| MPT/PHBV(B) | CHCl3/MeOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
526 ± 167 | 101.50 ± 0.61 | |
For electrospinning, the syringe loaded with the electrospinning solutions was fitted with 18 G blunted stainless steel needle and pumped at a controlled flow rate of 1 mL h−1 by a syringe pump (KDS 100, KD Scientific, and Holliston, MA) with a high voltage of 16 kV (Gamma High Voltage, USA) was applied. Nanofibers were collected on aluminum foil and 15 mm cover slips, and they were dried overnight under vacuum and used for drug release and cell proliferation experiments.
2.3 Characterization of nanofibers
2.4 In vitro drug release study
The drug loaded nanofibrous mats were cut into small pieces, and approximately 10 mg of sample was immersed in 1 mL of pH 7.4 phosphate buffer solution (PBS) at 37 °C. At predetermined time intervals, the PBS was completely removed from each sample for analysis and 1 mL of fresh PBS was refill for continuous incubation. This iterative process lasted for 21 days. The amount of drug released in PBS at each time point was monitored by measuring the UV absorbance of the maximum peak for MH (at an optical wavelength of 223 nm) and MPT (274 nm) with a Shimadzu UV-3600 spectrometer. The cumulated drug release ratio was calculated with the following formula:where Mt is the amount of drugs released at time t and M∞ is the total amount of drug incorporated theoretically into the nanofibers. The samples at all-time points were run in triplicate.
After 21 days drug release, all the drug loaded nanofibrous mats were kept in a fume hood for air drying overnight, and further observation of surface morphology were measured by FESEM test.
2.5 Cell culture and in vitro cytotoxicity study
Human MSCs were cultured in 75 cm2 flasks containing DMEM supplemented with 10% FBS and 1% antibiotic. The culture flasks were then incubated at 37 °C in a humidified atmosphere containing 5% CO2. After culturing a sufficient amount of cells, the cytotoxic effect of drug loaded nanofibers was studied using 3 day, 6 day and 9 day [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, MTS] assays. Briefly, the 15 mm cover slips with electrospun nanofibrous mats were sterilized under UV light, washed thrice with sterile PBS, subsequently immersed in culture medium for 30 min, then placed in 24-well plate and pressed with a stainless steel ring. Cells were detached by trypsin/EDTA, counted by haemocytometer and seeded on the nanofibrous mats at a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well. After 3, 6 and 9 days of incubation, cells were washed with PBS and incubated with pure DMEM containing 20% MTS reagent for 3 hours, and aliquots of every wells were pipetted into a 96-well plate. The absorbance of each well was measured at 490 nm with a Varioskan Flash microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). Cells incubated on blank cover slips, pure PCL and PHBV nanofibrous mats were used as controls.
000 cells per well. After 3, 6 and 9 days of incubation, cells were washed with PBS and incubated with pure DMEM containing 20% MTS reagent for 3 hours, and aliquots of every wells were pipetted into a 96-well plate. The absorbance of each well was measured at 490 nm with a Varioskan Flash microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). Cells incubated on blank cover slips, pure PCL and PHBV nanofibrous mats were used as controls.
2.6 Morphological properties of mesenchymal stem cells
The morphology of MSCs cultured on drug-loaded emulsion electrospun nanofibrous mats were observed by FESEM. After incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 6 days, all nanofibrous mats with cells were fixed with 3% glutaraldehyde for 3 h, then rinsed with distilled water and dehydrated using graded concentrations (50, 70, 90, 100% v/v) of ethanol. Subsequently the samples of the nanofibrous mats were treated with hexamethyldisilazane (HMDS), kept in a fume hood for air drying overnight and finally coated with gold for the observation of cell morphology.3. Results and discussion
Electrospinning of W/O emulsions is a complex process influenced mainly by the properties of emulsions, including emulsion stability, viscosity and conductivity.9,12,14,16 The properties of the emulsions can be modulated by varying the stabilizer, polymeric material used and the added drugs.9,25 Stabilizer, polymeric material and the added drug are also known as surfactants, oil phase and water phase in emulsions. In our previous work, we studied the influence of surfactants on the properties of PCL nanofibers, and found 1% Span 80 (v/v) and 0.4% sodium dodecyl sulfate (SDS) (w/v) are the most suitable surfactant and surfactant dosage for the preparation of optimized nanofibrous scaffolds.9 So far, systematic study on controlled drug release of the emulsion electrospun nanofibers is rare.In this study, we chose Span 80 as the surfactant to prepare stable emulsions. In order to understand the influence of oil phase and water phase on the release of a drug from emulsion electrospun nanofibers, two drugs and two polymers differing in their physicochemical properties were used for comparison, the drug release properties of blend electrospun nanofibers were also studied. The drug release study of PHBV nanofibers has rarely been investigated and this is the first study on the release properties of highly water-soluble drug loaded PHBV emulsion electrospun nanofibers. Furthermore, a systematic study on the influence of oil phase and water phase towards drug release behavior of the emulsion electrospun nanofibers has not been documented to date.
3.1 Electrospinning conditions of various nanofibers
Table 1 lists the experimental conditions used for various scaffolds. For the emulsion electrospinning process, CHCl3 was chosen as the sole solvent, while blend electrospinning was performed using CHCl3 and MeOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v). It was necessary to use a mixture of CHCl3 and MeOH for blend electrospinning, since our preliminary experiments used CHCl3 as the sole solvent resulting in beaded and non-uniform nanofibers. With the addition of small amounts of MeOH to CHCl3, the electrospinnability of blend solution was enhanced, producing nanofibers with better morphology.26 What's more, the hydrophilic drugs are normally immiscible in organic solvents but are sometimes freely soluble in methanol. Therefore, CHCl3/MeOH (80
20 v/v). It was necessary to use a mixture of CHCl3 and MeOH for blend electrospinning, since our preliminary experiments used CHCl3 as the sole solvent resulting in beaded and non-uniform nanofibers. With the addition of small amounts of MeOH to CHCl3, the electrospinnability of blend solution was enhanced, producing nanofibers with better morphology.26 What's more, the hydrophilic drugs are normally immiscible in organic solvents but are sometimes freely soluble in methanol. Therefore, CHCl3/MeOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) was selected to increase the spinnability of PHBV and the drug powders distributed well in blend spinning solutions. 15% (w/v) PHBV in CHCl3/MeOH was used for the production of pure PHBV and blend electrospinning PHBV nanofibers. While for the emulsion electrospinning of PHBV nanofibers, only 13% (w/v) PHBV in CHCl3 was required, which means, electrospinning of a dilute polymer solution can be achieved by emulsion electrospinning, thus maximizing the potential of electrospinning for tissue engineering and drug delivery applications.9 Therefore, emulsion electrospinning could be an effective strategy to prepare nanofibers from solutions at low concentrations, which make the experiment cost-effective, especially considering mass scale production.
20) was selected to increase the spinnability of PHBV and the drug powders distributed well in blend spinning solutions. 15% (w/v) PHBV in CHCl3/MeOH was used for the production of pure PHBV and blend electrospinning PHBV nanofibers. While for the emulsion electrospinning of PHBV nanofibers, only 13% (w/v) PHBV in CHCl3 was required, which means, electrospinning of a dilute polymer solution can be achieved by emulsion electrospinning, thus maximizing the potential of electrospinning for tissue engineering and drug delivery applications.9 Therefore, emulsion electrospinning could be an effective strategy to prepare nanofibers from solutions at low concentrations, which make the experiment cost-effective, especially considering mass scale production.
3.2 Characterization of nanofibrous scaffolds
The endothermic peak of drug is missing in the thermograms of MH loaded nanofibers by emulsion electrospinning and all the MPT incorporated nanofibers, which proves that MH in emulsion electrospun nanofibers and MPT in the emulsion and blend electrospun mats were present in an amorphous state. Our study demonstrated that the incorporation of small amounts of drugs and surfactants during electrospinning process did not significantly change the thermal properties of the drug loaded emulsion electrospun mats.
3.3 In vitro drug release study
In vitro release profile of MH and MPT from PCL and PHBV nanofibers was studied in phosphate buffer solution (pH 7.4) to evaluate the potential application of MH and MPT loaded nanofibers as drug delivery system. The cumulative release curve of the drug loaded nanofibers are shown in Fig. 5–7 where y-coordinate represent cumulative percent drug release and x-coordinate represent drug release time points. It is obviously that the drug release profile of all the drug loaded nanofibrous scaffolds can be split into two phases: an initial burst release, with a significant amount of drug released within 12 hours and a sustained drugs release pattern after 12 hours. From Fig. 5 to 7, it was obvious that the drug entrapped emulsion electrospun nanofibers exhibited a lower burst release and slower release speed compared with the blend electrospun nanofibers; drug–PHBV emulsion electrospun nanofibers revealed higher burst release and faster drug release speed than the drug–PCL emulsion electrospun nanofibers; MH and MPT encapsulated nanofibers showed a different release profiles.As shown in Fig. 5, the amount of drug released within 12 hours was 34.82% for MPT/PCL(E) and 67.77% for MPT/PCL(B) nanofibers, and the cumulative drug release over 2 days was 55% for MPT/PCL(E) and 82% for MPT/PCL(B) nanofibers, the cumulative release percentage of MPT over 21 days was 68% for MPT/PCL(E) and 95% for MPT/PCL(B) nanofibers, etc.
Former researchers have proved that hydrophilic drugs usually exhibit a tendency to migrate to the surface of nanofibers when they were embedded in hydrophobic polymers.35 In this study, the highly water-soluble drugs were simply mixed with the hydrophobic polymers during blend electrospinning process and most of the hydrophilic drugs may have a tendency to be trapped on the fibrous surface, leading to an extremely large burst release. While for emulsion electrospinning, the hydrophilic drug was firstly dissolved in distilled water with the addition of surfactants and then added dropwise to the polymer–chloroform solution, during this process drug molecules were wrapped by surfactants and polymer, and a core–shell structure was formed.36 The hydrophobic polymer (PCL or PHBV) formed the shell while the hydrophilic drug (MH or MPT) was gathered within aqueous phase of the core. The hydrophobic polymer shell separated drugs from the outer aqueous medium and hindered the entry of the dissolution medium into nanofibers. Since most of the drugs were in the core, lower and slower drug release rate were observed for emulsion electrospun nanofibers compared with blend electrospun nanofibers.
Fig. 6 exhibited that the drug–PHBV emulsion electrospun nanofibers revealed higher burst release and faster drug release rate than the drug–PCL emulsion electrospun nanofibers, which could be attributed to the different inherent properties of PHBV and PCL. Firstly, PHBV is a semi crystalline polymer with a high crystallinity (60–80%)37 and a relatively high melting point around 160 °C (Fig. 3). Due to the high crystallinity, PHBV might not be suitable to encapsulate hydrophilic drugs,38 and most of the drug molecules might have been located on the surface of nanofibers during the electrospinning process, leading to the fast drug released of both MH and MPT. However, PCL are rubbery materials with lower melting point (∼60 °C) and crystallinity (45–60%),39 making PCL more suitable as a matrix for drug delivery system than PHBV. Secondly, comparing the water contact angles, pure PHBV appeared more hydrophilic than pure PCL, and hence the drug–PHBV nanofibers had a higher permeability to surrounding PBS, making drug molecules diffused rapidly through PHBV nanofibers. On the contrary, the relatively hydrophobic nature of PCL retarded PBS solution influx thus limiting drug diffusion, contributing to slower drug release rates from drug loaded PCL nanofibers. Thirdly, for hydrophilic drugs, the release rate is modulated mainly by the steric interaction between the drug molecule and the matrix.40 And PHBV and PCL are quite different in physical–chemical properties, which might result in the variance steric interaction between the drug molecule and the polymer matrix.
Fig. 7 shows the influence of drug characteristics on drug release profiles of MH or MPT incorporated nanofibers while being prepared by same techniques and polymers. Obviously, MH released slightly faster than MPT, especially when PHBV was used as drug deliver matrix. In detail, the amount of drug released within 12 hours was 74% for MH/PHBV(E), 71% for MPT/PHBV(E), 86% from MH/PHBV(B) and 77% for MPT/PHBV(B). These results could be explained by the differences in molecular weights and hydrophilicity of these two drugs. MH is a highly hydrophilic drug with smaller molecular weight (Mw 165.62), while MPT is less hydrophilic than MH and have a larger molecular weight (Mw 684.81). The faster release of MH could be due to its higher water-solubility than MPT,20,35 as several researchers have found that a hindrance of rapid drugs dissolution can be gained due to limited drug solubility.41 Also, drug size is another parameter defining the release kinetics. A bigger size of drug can alleviate burst release and contribute to the sustained release of drug.42
In summary, the different release profiles of MH and MPT encapsulated nanofibers are related to their distribution and aggregates within the nanofibers, influenced by the preparation techniques (emulsion or blend electrospinning), polymer matrix (PCL or PHBV) and the drug physicochemical properties. PCL could be a more effective carrier for drug delivery system than PHBV.
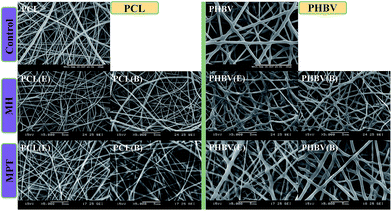
It is generally believed that drug release from electrospun polymeric nanofibers consist of several processes, including diffusion through the polymer matrix,1 release by polymer degradation,43 and the desorption of the drugs from the nano-porous surface of the nanofibers.44 The solute transport from biodegradable polymeric systems is mainly considered as diffusion driven.12 To match the assumptions of diffusion controlled release kinetics, the initial drug concentration in the release system should be much higher than the drug solubility.45 However, in the current study, the theoretical concentrations of both two drugs within the nanofibers were well below their water solubility limit. In addition, Fig. 8 shows the morphologies of blend and emulsion electrospun nanofibers after 21 days drug release, where fiber breakages were only observed from drug incorporated PHBV nanofibers. Therefore, the release kinetic cannot be simply classified as diffusion controlled kinetic.
 | ||
| Fig. 8 FESEM images of blend electrospun and emulsion electrospun nanofibrous scaffolds after 21 days release in PBS. Note: fiber breakages are indicated within PHBV nanofibers. | ||
It would be worthwhile taking polymer degradation (for PHBV nanofibers) and burst release into consideration to better predict the drug release kinetics. So the possible release kinetics of the drug–PCL nanofiber drug delivery systems consist of two dominant driving forces, namely, drug desorption and diffusion controlled release kinetics. The initial burst release of drugs in stage I (0–12 h) would be related to the rapid desorption and diffusion of the drugs from nanopores within the surface vicinity of the nanofibers as soon as the nanofibers were placed in the aqueous medium. Further drug diffusion followed from the inner part of matrix (the second stage). In the second stage (12–504 h), the drugs would preferably diffuse out through the near surface or inner surface nanopores, and these nanopores may penetrate deep inside the PCL nanofibers, most probably forming an interconnected network. In the current study, the second phase release (12–504 h) could be divided into two parts, in the first part (12–48 h), drugs diffused from the shallow part of the polymer matrix quickly, while 48 h to 504 h represented the second part, as drugs diffused from within the deeper regions of the delivery systems by means of aqueous channels of a network of nanopores. According to Fig. 8, the drug loaded PCL nanofibers remained stable and were not degraded or corroded after 21 days, owing to the hydrophobic properties and long degradation time (>2 years for complete degradation) of PCL. However, as stated above, pure PHBV nanofibers are more hydrophilic than PCL nanofibers, leading to the faster degradation of drug–PHBV nanofibers than drug–PCL nanofibers, which in turn influence the release properties of the drug–PHBV nanofibrous scaffolds and end up in increasing drug release rate from drug–PHBV nanofibers.
3.4 In vitro cytotoxicity study of electrospun nanofibers
To study the cytotoxicity of electrospun nanofibers, the [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, MTS] assay was performed. The MTS assay is an important method for evaluating the in vitro cytotoxicity of biomaterials. The principle of this assay is that in the presence of phenazine methosulfate (PMS), the yellow tetrazolium salt in MTS is reduced to purple formazan by the dehydrogenase enzymes in living cells. The amount of formazan produced is directly proportional to the number of live cells. And the formazan product has an absorbance maximum at 490–500 nm in phosphate-buffered saline.From a clinical point of view, an ideal drug delivery system must have a good compatibility with tissue cells and a sustained controlled drug release. In present study, all drug-loaded blend electrospun nanofibers showed much lower hydrophilicity and a higher drug burst release compared with all emulsion electrospun nanofibers. Therefore, the blend electrospun nanofibers are not suitable as the controlled drug delivery system. The cytotoxicity of drug loaded nanofibers was studied only using emulsion electrospun nanofibers. Fig. 9 depicts the MTS assay profile of MSC proliferation on pure polymer nanofibers, drug–polymer emulsion electrospun nanofibers and cover slips (TCP). MTS assay showed that the number of live cell in all scaffolds increased with the prolonging of incubation time, and among all the scaffolds, MPT–PCL(E) nanofibers have increased the most number of live cells and MH–PHBV(E) nanofibers showed the least live cell numbers. Also, the MTS assay exhibited that no significant difference was observed in the number of cells on day 3; by day 9, the number of cell viability on MPT–PCL(E) surpassed that on TCP and ranked first, while the MH–PHBV(E) scaffolds showed the least cell viability as always. Fig. 10 shows the electron micrographs of hMSC on all materials after 6 days of cultured. The morphologies of hMSC proliferation on all nanofibers and TCP were consistent with the results of the MTS assay. The results of the current work demonstrate that the pure PCL and PHBV nanofibers, drug–PCL and drug–PHBV emulsion electrospun nanofibers have no visible cytotoxicity against hMSCs, that the drug–polymer emulsion electrospun nanofibers have a good bio-compatibility with tissue cells, and cell proliferation on MPT incorporated emulsion electrospun nanofibers were much better than that on MH loaded nanofibers. These results reveal that the incorporation of tartrate salt has not caused any adverse effects on cell proliferation.46 And the presence of hydrochloride part in MH molecules might have negative effective on cell proliferation, leading to lower cell viability,47,48 suggesting that the MPT–PCL(E) scaffold has the potential for biomedical application.
 | ||
| Fig. 10 Cell morphology on the drug loaded emulsion electrospun nanofibers compared to the control scaffolds and TCP on day 6. | ||
4. Conclusion
MH and MPT incorporated nanofibers were prepared by using either PCL or PHBV as the polymer matrix by emulsion and blend electrospinning technique. The wettability study showed that the emulsions electrospinning technique can help improve the hydrophilicity of nanofibers from hydrophobic polymers substrates. Compared to the blend electrospun nanofibers, emulsion electrospun nanofibers suppressed the initial burst release and released drugs continuously. The drug–PCL emulsion electrospun nanofibers revealed a lower and slower drug release than the drug–PHBV emulsion electrospun nanofibers and the MPT–PCL(E) nanofibers exhibited the lowest burst release and slowest drug release, suggesting PCL the most suitable drug delivery matrix compared to PHBV. In vitro cytotoxicity study demonstrated that the drug–polymer emulsion electrospun nanofibers had no cytotoxicity and were biocompatible, and that the MPT–PCL(E) nanofibers exhibited superior ability for MSCs adhesion and has potential for biomedical application. Our results suggest that emulsion electrospinning technology is a promising technique to produce core–shell nanofibers for the design of controlled-release drug delivery systems, and that the drug release rate could be modulated by adjusting the oil phase and water phase.Acknowledgements
Ms Jue Hu thanks the China Scholarship Council for providing the financial support to help her perform the present research work in NUS. This work was supported through a grant from the Dept. of Mechanical Engineering, Faculty of Engineering, National University of Singapore, Singapore, NRF-Technion [grant number R-265-000-538-592].References
- X. L. Xu, X. S. Chen, Z. F. Wang and X. B. Jing, Eur. J. Pharm. Biopharm., 2009, 72, 18–25 CrossRef CAS PubMed.
- Y. Liao, L. Zhang, Y. Gao, Z. T. Zhu and H. Fong, Polymer, 2008, 49, 5294–5299 CrossRef CAS PubMed.
- R. Qi, R. Guo, M. Shen, X. Cao, L. Zhang, J. Xu, J. Yu and X. Shi, J. Mater. Chem., 2010, 20, 10622–10629 RSC.
- S. Wang, F. Zheng, Y. Huang, Y. Fang, M. Shen, M. Zhu and X. Shi, ACS Appl. Mater. Interfaces, 2012, 4, 6393–6401 CAS.
- F. Zheng, S. Wang, M. Shen, M. Zhu and X. Shi, Polym. Chem., 2013, 4, 933–941 RSC.
- F. Zheng, S. Wang, S. Wen, M. Shen, M. Zhu and X. Shi, Biomaterials, 2013, 34, 1402–1412 CrossRef CAS PubMed.
- S. Maretschek, A. Greiner and T. Kissel, J. Controlled Release, 2008, 127, 180–187 CrossRef CAS PubMed.
- Y. Yang, X. H. Li, M. B. Qi, S. B. Zhou and J. Weng, Eur. J. Pharm. Biopharm., 2008, 69, 106–116 CrossRef CAS PubMed.
- J. Hu, M. P. Prabhakaran, X. Ding and S. Ramakrishna, J. Biomater. Sci., Polym. Ed., 2014, 26, 57–75 CrossRef PubMed.
- M. W. Lee, S. An, C. Lee, M. Liou, A. L. Yarin and S. S. Yoon, ACS Appl. Mater. Interfaces, 2014, 6, 10461–10468 CAS.
- L. Zhou, C. Zhu, L. Edmonds, H. Yang, W. Cui and B. Li, RSC Adv., 2014, 4, 43220–43226 RSC.
- X. L. Xu, X. S. Chen, P. A. Ma, X. R. Wang and X. B. Jing, Eur. J. Pharm. Biopharm., 2008, 70, 165–170 CrossRef CAS PubMed.
- L. Tian, M. P. Prabhakaran, X. Ding, D. Kai and S. Ramakrishna, J. Mater. Sci., 2011, 47, 3272–3281 CrossRef.
- Y. Yang, T. Xia, W. Zhi, L. Wei, J. Weng, C. Zhang and X. H. Li, Biomaterials, 2011, 32, 4243–4254 CrossRef CAS PubMed.
- L. Tian, M. Prabhakaran, X. Ding, D. Kai and S. Ramakrishna, J. Mater. Sci.: Mater. Med., 2013, 24, 2577–2587 CrossRef CAS PubMed.
- L. Tian, M. P. Prabhakaran, X. Ding and S. Ramakrishna, J. Biomater. Sci., Polym. Ed., 2013, 24, 1952–1968 CrossRef CAS PubMed.
- X. Xu, X. Zhuang, X. Chen, X. Wang, L. Yang and X. Jing, Macromol. Rapid Commun., 2006, 27, 1637–1642 CrossRef CAS.
- M. K. Riekes, F. M. Barboza, D. D. Vecchia, M. Bohatch Jr, P. V. Farago, D. Fernandes, M. A. S. Silva and H. K. Stulzer, Mater. Sci. Eng., C, 2011, 31, 962–968 CrossRef CAS.
- F. M. Barboza, W. M. Machado, L. R. Olchanheski Junior, J. Padilha de Paula, S. F. Zawadzki, D. Fernandes and P. V. Farago, Sci. World J., 2014, 2014, 1–10 CrossRef PubMed.
- W. L. Lee, P. Wee, C. Nugraha and S. C. J. Loo, J. Mater. Chem. B, 2013, 1, 1090–1095 RSC.
- W. S. N. Shim, S. Jiang, P. Wong, J. Tan, Y. L. Chua, Y. Seng Tan, Y. K. Sin, C. H. Lim, T. Chua, M. Teh, T. C. Liu and E. Sim, Biochem. Biophys. Res. Commun., 2004, 324, 481–488 CrossRef CAS PubMed.
- A. J. Nauta and W. E. Fibbe, Blood, 2007, 110, 3499–3506 CrossRef CAS PubMed.
- X. Wei, X. Yang, Z.-P. Han, F.-F. Qu, L. Shao and Y.-F. Shi, Acta Pharmacol. Sin., 2013, 34, 747–754 CrossRef CAS PubMed.
- X. Q. Li, Y. Su, X. Zhou and X. M. Mo, Colloids Surf., B, 2009, 69, 221–224 CrossRef CAS PubMed.
- M. A. Badawi and L. K. El-Khordagui, Eur. J. Pharm. Sci., 2014, 58, 44–54 CrossRef CAS PubMed.
- S. Gautam, A. K. Dinda and N. C. Mishra, Mater. Sci. Eng., C, 2013, 33, 1228–1235 CrossRef CAS PubMed.
- C. Sander, H. M. Nielsen and J. Jacobsen, Int. J. Pharm., 2013, 458, 254–261 CrossRef CAS PubMed.
- D. Myers, in Surfactant Science and Technology, John Wiley & Sons, Inc., 2005, pp. 1–28 Search PubMed.
- A. Vogel and J. Mendham, Vogel's textbook of quantitative chemical analysis, John Wiley & Sons, Inc., USA, 1989 Search PubMed.
- A. Bright, T. R. Devi and S. Gunasekaran, Int. J. ChemTech Res., 2010, 2, 379–388 CAS.
- W. Chen and Y. W. Tong, Acta Biomater., 2012, 8, 540–548 CrossRef CAS PubMed.
- S. K. Tiwari, R. Tzezana, E. Zussman and S. S. Venkatraman, Int. J. Pharm., 2010, 392, 209–217 CrossRef CAS PubMed.
- K. J. Wadher, R. B. Kakde and M. J. Umekar, Int. J. Pharm. Invest., 2011, 1, 157–163 CrossRef CAS PubMed.
- D.-C. Marinescu, E. Pincu and V. Meltzer, Int. J. Pharm., 2013, 448, 366–372 CrossRef CAS PubMed.
- E.-R. Kenawy, F. I. Abdel-Hay, M. H. El-Newehy and G. E. Wnek, Mater. Chem. Phys., 2009, 113, 296–302 CrossRef CAS.
- A. Szentivanyi, T. Chakradeo, H. Zernetsch and B. Glasmacher, Adv. Drug Delivery Rev., 2011, 63, 209–220 CrossRef CAS PubMed.
- C. W. Pouton and S. Akhtar, Adv. Drug Delivery Rev., 1996, 18, 133–162 CrossRef CAS.
- J. B. E. Mendes, M. K. Riekes, V. M. de Oliveira, M. D. Michel, H. K. Stulzer, N. M. Khalil, S. F. Zawadzki, R. M. Mainardes and P. V. Farago, Sci. World J., 2012, 2012, 1–13 CrossRef PubMed.
- M. J. Jenkins and K. L. Harrison, Polym. Adv. Technol., 2008, 19, 1901–1906 CrossRef CAS.
- G. Fundueanu, M. Constantin and P. Ascenzi, Acta Biomater., 2009, 5, 363–373 CrossRef CAS PubMed.
- Y. Z. Zhang, X. Wang, Y. Feng, J. Li, C. T. Lim and S. Ramakrishna, Biomacromolecules, 2006, 7, 1049–1057 CrossRef CAS PubMed.
- A. Bertz, S. Wöhl-Bruhn, S. Miethe, B. Tiersch, J. Koetz, M. Hust, H. Bunjes and H. Menzel, J. Biotechnol., 2013, 163, 243–249 CrossRef CAS PubMed.
- L. L. Lao, N. A. Peppas, F. Y. C. Boey and S. S. Venkatraman, Int. J. Pharm., 2011, 418, 28–41 CrossRef CAS PubMed.
- R. Srikar, A. L. Yarin, C. M. Megaridis, A. V. Bazilevsky and E. Kelley, Langmuir, 2008, 24, 965–974 CrossRef CAS PubMed.
- J. Siepmann and N. A. Peppas, Adv. Drug Delivery Rev., 2001, 48, 139–157 CrossRef CAS PubMed.
- T. Peters, R. Fulton, K. Roberson and M. Orth, Avian Dis., 2002, 46, 75–86 CrossRef CAS PubMed.
- E. Obłąk and A. Krasowska, Adv. Clin. Exp. Med., 2010, 19, 65–75 Search PubMed.
- H. Mellegård, S. P. Strand, B. E. Christensen, P. E. Granum and S. P. Hardy, Int. J. Food Microbiol., 2011, 148, 48–54 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |