Assembly of BF4−, PF6−, ClO4− and F− with trinuclear copper(I) acetylide complexes bearing amide groups: structural diversity, photophysics and anion binding properties†
Hua-Yun Shi,
Yong-Liang Huang,
Jia-Kai Sun,
Ji-Jun Jiang,
Zhi-Xing Luo,
Hui-Tao Ling,
Chi-Keung Lam and
Hsiu-Yi Chao*
MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou 510275, P. R. China. E-mail: zhaoxy@mail.sysu.edu.cn; Fax: +86-20-84112245; Tel: +86-20-84110062
First published on 15th October 2015
Abstract
Trinuclear copper(I) acetylide complexes 1·BF4–4·BF4, 1·PF6, 1·ClO4 and 4·F have been synthesized and characterized. Five kinds of discrete or polymeric structures could be found in their crystal structures. Among them, complexes 1·BF4, 1·PF6, and 1·ClO4 form zigzag one-dimensional (1D) anion coordination polymers (ACPs) using anions as nodes and cations 1 as ligands. For complex 2·BF4, hydrogen bonds between adjacent amide groups afford the zigzag 1D polymeric chains, which are supported by the interaction between dppms and anions. A 1D infinite meso-helical hydrogen bonding polymeric chain with a counter anion bound in each cation can be observed in complex 3·BF4. Complex 4·BF4 is unable to form polymeric chains, while complex 4·F that exhibits similar structure with 4·BF4 could construct infinite 1D polymer via hydrogen bonds between amide groups. The photophysical properties of copper(I) acetylide complexes have been studied. They show luminescence both in the solid state and DMSO solution at 298 K. The anion binding abilities of complexes 1·BF4–4·BF4 in DMSO have also been studied by using 1H NMR and UV-vis titration experiments. Their dramatic color change towards F− in DMSO enables naked eye detection of F−.
Introduction
Anion coordination chemistry has attracted growing interest and developed rapidly in recent years, owing to the chemical, biological and environmental importance of anions.1–8 In 1968, Park and Simmons9 reported the first hydrogen bonding based halide sensor, which was regarded as the herald of anion coordination chemistry. The concept of anion coordination was firstly proposed by Lehn10 in 1978, which exhibited remarkable similarities and differences with traditional metal coordination chemistry. Compared with metal coordination, anion coordination is formed via the weak interactions (mainly hydrogen bond) between anions and ligands, rather than the covalent bond between ligands and metals. Owing to the diverse range of sizes, geometries, basicities and hydrogen-bonding modes of different anions, complexation of anions with the receptor molecules is highly challenging and requires delicate designs of host molecules.11 The groups of Beer,12–15 Gale,16–19 Steed,20–23 Custelcean,24–27 Wu28–32 and others33–36 have reported novel anion-based architectures and studied their anion-binding modes, anion separation properties and fluorescence properties. Notably, supramolecular architectures with anions as the coordination nodes and organic ligand or metal complexes as linkers, bearing infinite polymeric nature, are defined as anion coordination polymer (ACPs) and yet to be explored.17,18,21,27,28,37 In contrast to the well-developed metal coordination polymers (CPs), in which metals with specific geometrical preference are employed as nodes, the construction of ACPs is imposed with more difficulties due to the weaker bonding strength of hydrogen bond and higher complexity of anions.11 ACPs not only have potential applications as sensors28,37 or optical materials,32,37 but also exhibit structural and topological novelty with diverse and interesting structural motifs. The simplest type of ACPs, 1D-ACPs, which usually bear properties38–39 such as anion exchange, gelation, and nanocrystal synthetic template, exhibit diverse polymeric architectures38 such as linear, zigzag, helical, and ladder. The key factors that could affect the structures of ACPs are building blocks: metal, ligands, and counter anions.28,40 Previous works included sulfate directed double strand helical self-assembly of chiral bicyclic guanidinium tetramers firstly reported by Mendoza's group,41 1D linear ACPs with acetate or terephthalate carboxylate anions and a bis-bisurea ligand based on a biphenyl backbone published by Gale's group,17 chloride bridged supramolecular polymeric network with BF2 complexes of acyclic dipyrrolyldiketone constructed by Maeda's group,37 and a series of ACPs with a bis-bisurea ligand that bears a rigid naphthylene spacer as a linker between two anions, including SO42−, AcO−, p-[COO–C6H4–COO]2−, Cl−, and Br−, studied by Wu's group.28 To date, however, anion-templated polymeric assemblies are very rare, and most of well-studied structures employed organic receptors as linkers.40In the past decades, metal complexes have been frequently used as anion sensors due to their properties like redox and luminescence, which could provide various accesses of sensing.42 Trinuclear copper(I) acetylide complexes have attracted considerable attention because of their rich photophysical and photochemical properties.43–47 The first trinuclear copper(I) complex with two capped μ3-η1-acetylides with short Cu(I)⋯Cu(I) distances, [Cu3(μ-dppm)3(μ3-η1-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)2](BF4) (dppm = bis(diphenylphosphino)methane), was reported by Gimeno and co-workers48 in 1991. Subsequently, a systematical investigation on the photophysical properties of bicapped trinuclear copper(I) acetylide complexes was performed by Yam's group.43–47 Our group49 also reported a series of trinuclear copper(I) acetylide complexes bearing carbonyl moiety. However, research on trinuclear copper(I) acetylide complexes as anion sensors is still blank.
CPh)2](BF4) (dppm = bis(diphenylphosphino)methane), was reported by Gimeno and co-workers48 in 1991. Subsequently, a systematical investigation on the photophysical properties of bicapped trinuclear copper(I) acetylide complexes was performed by Yam's group.43–47 Our group49 also reported a series of trinuclear copper(I) acetylide complexes bearing carbonyl moiety. However, research on trinuclear copper(I) acetylide complexes as anion sensors is still blank.
Neutral N–H or cationic (N–H)+ hydrogen bond donor is a key component of anion receptors, and amide-based ligands belong to the neutral-type anion receptors.50 It is interesting to note that anion binding by proteins is mostly achieved by way of neutral amide functional groups.51 The highly accessible hydrogen-bond donor with directional hydrogen-bonding being involved for the host–guest interaction11 provides amide receptors with a differentiating power to screen anions of different geometries or hydrogen bonding requirements.52 These features, combined with their simple structures and easy modification by organic synthesis, make amide groups commonly be employed in the design of anion sensors. The supporting interactions between appropriately placed backbone C–H protons and anions are essential as well and enhance the anion-binding affinity.22,53–56 In some cases, C–H sites associate with anions without the supporting N–H.53–56 Theoretical studies also support the interactions between C–H units and anions.57–58 Aromatic C–Hs involve in the interaction with anions most frequently, while Maeda's group37 reported the first example of anion recognition assisted by nonaromatic C–H⋯anion interactions.
Transition metal complexes with amide N–H hydrogen bond donor as anion-binding sites are our group's long-term interest.59 Complexes with different R substituents exhibit varied affinities toward anions compared with their analogues,59 we therefore envisaged that the acidity of amide groups could influence their coordination patterns and selective crystallization to anions. In this work, we have synthesized and characterized a series of trinuclear copper(I) acetylide complexes, [Cu3(μ-dppm)3(μ3-η1-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-4-NHC(O)C6H4-4-R)2]BF4 (R = NO2 (1·BF4), H (3·BF4) and OCH3 (4·BF4)), [Cu3(μ-dppm)3(μ3-η1-C
CC6H4-4-NHC(O)C6H4-4-R)2]BF4 (R = NO2 (1·BF4), H (3·BF4) and OCH3 (4·BF4)), [Cu3(μ-dppm)3(μ3-η1-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-4-NHC(O)C6H4-4-CF3)(μ2-η1-C
CC6H4-4-NHC(O)C6H4-4-CF3)(μ2-η1-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-4-NHC(O)C6H4-4-CF3)]BF4 (2·BF4), [Cu3(μ-dppm)3(μ3-η1-C
CC6H4-4-NHC(O)C6H4-4-CF3)]BF4 (2·BF4), [Cu3(μ-dppm)3(μ3-η1-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-4-NHC(O)C6H4-4-NO2)2]X (X = PF6 (1·PF6) and ClO4 (1·ClO4)), and [Cu3(μ-dppm)3(μ3-η1-C
CC6H4-4-NHC(O)C6H4-4-NO2)2]X (X = PF6 (1·PF6) and ClO4 (1·ClO4)), and [Cu3(μ-dppm)3(μ3-η1-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-4-NHC(O)C6H4-4-OCH3)2]F (4·F). The X-ray crystal structures of anion complexes 1·BF4–4·BF4, 1·PF6, 1·ClO4 and 4·F were analyzed in detail to investigate the influence of anions as well as R substituents on polymeric architectural diversity. The photophysics of acetylide ligands and complexes as well as anion binding properties of complexes 1·BF4–4·BF4 in DMSO solution were also studied.
CC6H4-4-NHC(O)C6H4-4-OCH3)2]F (4·F). The X-ray crystal structures of anion complexes 1·BF4–4·BF4, 1·PF6, 1·ClO4 and 4·F were analyzed in detail to investigate the influence of anions as well as R substituents on polymeric architectural diversity. The photophysics of acetylide ligands and complexes as well as anion binding properties of complexes 1·BF4–4·BF4 in DMSO solution were also studied.
Results and discussion
Syntheses and characterization
The synthetic route of trinuclear copper(I) acetylide complexes 1·BF4–4·BF4, 1·PF6 and 1·ClO4 is summarized in Scheme 1. Acetylide ligands L1–L4 were obtained by using similar methods reported in our previous paper.59 The reactions of dinuclear complexes [Cu2(μ-dppm)2(CH3CN)4](X)2 (X = BF4−, PF6−, or ClO4−) with L1–L4 in the molar ration of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in degassed acetonitrile in the presence of triethylamine at 298 K gave trinuclear copper(I) acetylide complexes 1·BF4–4·BF4, 1·PF6 and 1·ClO4, respectively. All copper(I) acetylide complexes 1·BF4–4·BF4, 1·PF6 and 1·ClO4 are air-stable in the solid state at 298 K and can be well dissolved in CH2Cl2, CH3CN, THF and DMSO. They gave satisfactory elemental analysis and were all characterized by IR, ESI-MS and NMR.
4 in degassed acetonitrile in the presence of triethylamine at 298 K gave trinuclear copper(I) acetylide complexes 1·BF4–4·BF4, 1·PF6 and 1·ClO4, respectively. All copper(I) acetylide complexes 1·BF4–4·BF4, 1·PF6 and 1·ClO4 are air-stable in the solid state at 298 K and can be well dissolved in CH2Cl2, CH3CN, THF and DMSO. They gave satisfactory elemental analysis and were all characterized by IR, ESI-MS and NMR.
 | ||
| Scheme 1 Synthetic route for trinuclear copper(I) acetylide complexes 1·BF4–4·BF4, 1·PF6, and 1·ClO4. | ||
The IR spectra of the trinuclear copper(I) acetylide complexes 1·BF4–4·BF4, 1·PF6, and 1·ClO4 contain three bands at ca. 3368–3399, 2170–2270 and ca. 1656–1677 cm−1, which could be ascribed to ν(N–H), ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) and ν(C
C) and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of acetylide ligands, respectively. In the 1H NMR spectra, complexes 1·BF4–4·BF4, 1·PF6 and 1·ClO4 in CD3CN display a singlet at δ 8.90–9.30 ppm, which are assigned as the resonances of the amide N–H of the acetylide ligand. The chemical shifts of these peaks are in the following order: 1·BF4 > 2·BF4 > 3·BF4 > 4·BF4, which is in line with the decreasing of the electron-withdrawing ability of substituent R (R = NO2 (1), CF3 (2), H (3), OCH3 (4)) on the acetylide ligand. In addition, the chemical shifts at δ 6.64–8.54 ppm are attributed to the resonances of the protons on the aromatic rings of the dppm and acetylide ligands. A singlet at ca. δ 3.30 ppm is ascribed as the resonance of the protons of CH2 moieties on dppm ligands. The 31P NMR spectra of the complexes 1·BF4–4·BF4, 1·PF6 and 1·ClO4 in CD3CN show a singlet at ca. δ −6.00 ppm. While complex 1·PF6 in CD3CN shows an additional quintet at ca. δ −144.65 ppm, which can be ascribed to the counter anion PF6−. As for 19F NMR spectra in CD3CN, complexes 1·BF4–4·BF4 display two singlet at ca. δ −151.65 and −151.70 ppm with a proportion of 1
O) of acetylide ligands, respectively. In the 1H NMR spectra, complexes 1·BF4–4·BF4, 1·PF6 and 1·ClO4 in CD3CN display a singlet at δ 8.90–9.30 ppm, which are assigned as the resonances of the amide N–H of the acetylide ligand. The chemical shifts of these peaks are in the following order: 1·BF4 > 2·BF4 > 3·BF4 > 4·BF4, which is in line with the decreasing of the electron-withdrawing ability of substituent R (R = NO2 (1), CF3 (2), H (3), OCH3 (4)) on the acetylide ligand. In addition, the chemical shifts at δ 6.64–8.54 ppm are attributed to the resonances of the protons on the aromatic rings of the dppm and acetylide ligands. A singlet at ca. δ 3.30 ppm is ascribed as the resonance of the protons of CH2 moieties on dppm ligands. The 31P NMR spectra of the complexes 1·BF4–4·BF4, 1·PF6 and 1·ClO4 in CD3CN show a singlet at ca. δ −6.00 ppm. While complex 1·PF6 in CD3CN shows an additional quintet at ca. δ −144.65 ppm, which can be ascribed to the counter anion PF6−. As for 19F NMR spectra in CD3CN, complexes 1·BF4–4·BF4 display two singlet at ca. δ −151.65 and −151.70 ppm with a proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in peak area, which could be ascribed to BF4− (the natural abundance of 10B to 11B is 1/4). Complex 1·PF6 shows a doublet at δ −73.53 ppm with a coupling constant of 700 Hz, owing to 31P–19F coupling. Complex 4·F was obtained by addition of excess amount of F− into 4·BF4 in CH3CN. The IR, ESI-MS, 1H NMR and 31P NMR spectra of 4·F are similar to those of 4·BF4.
4 in peak area, which could be ascribed to BF4− (the natural abundance of 10B to 11B is 1/4). Complex 1·PF6 shows a doublet at δ −73.53 ppm with a coupling constant of 700 Hz, owing to 31P–19F coupling. Complex 4·F was obtained by addition of excess amount of F− into 4·BF4 in CH3CN. The IR, ESI-MS, 1H NMR and 31P NMR spectra of 4·F are similar to those of 4·BF4.
X-ray crystal structure
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR′)2]+ analogues,43–49 which consists of an approximately isosceles triangle of copper atoms with a dppm ligand bridging each edge to form a roughly planar [Cu3P6] core. The distances between two copper atoms are in the range of 2.5374(8)–2.7672(8) Å, which are shorter than the sum of van der Waals radii for copper atoms (2.8 Å).60 This observation suggests the presence of weak Cu⋯Cu interactions. Three Cu2P2C rings adopt envelope conformations with the methylene carbon atoms on the flap. One of them folds toward one of the faces of the Cu3 triangles, while the other two fold away from it. The Cu–P distances are in the range of 2.2549(12)–2.2948(11) Å, which resemble those in analogous trinuclear copper(I) acetylide complexes.43–49 Two C
CR′)2]+ analogues,43–49 which consists of an approximately isosceles triangle of copper atoms with a dppm ligand bridging each edge to form a roughly planar [Cu3P6] core. The distances between two copper atoms are in the range of 2.5374(8)–2.7672(8) Å, which are shorter than the sum of van der Waals radii for copper atoms (2.8 Å).60 This observation suggests the presence of weak Cu⋯Cu interactions. Three Cu2P2C rings adopt envelope conformations with the methylene carbon atoms on the flap. One of them folds toward one of the faces of the Cu3 triangles, while the other two fold away from it. The Cu–P distances are in the range of 2.2549(12)–2.2948(11) Å, which resemble those in analogous trinuclear copper(I) acetylide complexes.43–49 Two C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C groups bridge the Cu3 planar through an asymmetric μ3–η1 bridging mode with different Cu–C distances in the range of 2.077(4)–2.411(4) Å (for 2 exclusively, one of the C
C groups bridge the Cu3 planar through an asymmetric μ3–η1 bridging mode with different Cu–C distances in the range of 2.077(4)–2.411(4) Å (for 2 exclusively, one of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C group employs an asymmetric μ2–η1 bridging mode). It is noted that one of the three Cu–C distances is relatively longer than the other two Cu–C distances. The bond angles between the acetylide ligands and copper atoms in cation 1 are in the range of 123.0(4)–159.1(4)°. The C
C group employs an asymmetric μ2–η1 bridging mode). It is noted that one of the three Cu–C distances is relatively longer than the other two Cu–C distances. The bond angles between the acetylide ligands and copper atoms in cation 1 are in the range of 123.0(4)–159.1(4)°. The C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond distances are 1.197(6) and 1.203(6) Å, respectively, characteristic of typical metal acetylide σ bonding.61 The conformations of two acetylide motifs attached on Cu3 are not exactly identical to each other, which could be deduced from their different torsion angles. In the first acetylide motif, the torsion angles of C(5)–C(6)–N(1)–C(9) and C(15)–C(10)–C(9)–N(1) are 4.00° and 24.41°, respectively. In the other motif, the torsion angles of C(22)–C(21)–N(3)–C(24) and C(26)–C(25)–C(24)–N(3) are 16.37° and 30.22°, respectively. Dihedral angle between plane O(1)–C(9)–N(1) and plane O(4)–C(24)–N(3) is 27.48°, suggesting two amide moieties point to different directions. The C
C bond distances are 1.197(6) and 1.203(6) Å, respectively, characteristic of typical metal acetylide σ bonding.61 The conformations of two acetylide motifs attached on Cu3 are not exactly identical to each other, which could be deduced from their different torsion angles. In the first acetylide motif, the torsion angles of C(5)–C(6)–N(1)–C(9) and C(15)–C(10)–C(9)–N(1) are 4.00° and 24.41°, respectively. In the other motif, the torsion angles of C(22)–C(21)–N(3)–C(24) and C(26)–C(25)–C(24)–N(3) are 16.37° and 30.22°, respectively. Dihedral angle between plane O(1)–C(9)–N(1) and plane O(4)–C(24)–N(3) is 27.48°, suggesting two amide moieties point to different directions. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O distances for 1 are 1.202(6) and 1.214(7) Å, which resemble typical carbonyl groups in analogues amide receptors.50,59
O distances for 1 are 1.202(6) and 1.214(7) Å, which resemble typical carbonyl groups in analogues amide receptors.50,59
| Cu(1)⋯Cu(2) | 2.5653(8) |
| Cu(1)⋯Cu(3) | 2.7672(8) |
| Cu(2)⋯Cu(3) | 2.5374(8) |
| Cu(1)–C(1) | 2.175(4) |
| Cu(2)–C(1) | 2.077(4) |
| Cu(3)–C(1) | 2.411(4) |
| Cu(1)–C(16) | 2.310(4) |
| Cu(2)–C(16) | 2.114(4) |
| Cu(3)–C(16) | 2.135(4) |
| Cu(1)–P(4) | 2.2914(12) |
| Cu(1)–P(5) | 2.2616(13) |
| Cu(2)–P(2) | 2.2824(11) |
| Cu(2)–P(3) | 2.2832(11) |
| Cu(3)–P(1) | 2.2948(11) |
| Cu(3)–P(6) | 2.2549(12) |
| C(1)–C(2) | 1.197(6) |
| C(16)–C(17) | 1.203(6) |
| C(9)–O(1) | 1.214(7) |
| C(24)–O(4) | 1.202(6) |
| N(2)–O(2) | 1.218(7) |
| N(2)–O(3) | 1.210(7) |
| N(4)–O(5) | 1.221(7) |
| N(4)–O(6) | 1.223(7) |
| Cu(1)–C(1)–C(2) | 124.3(4) |
| Cu(2)–C(1)–C(2) | 159.1(4) |
| Cu(3)–C(1)–C(2) | 123.0(4) |
| Cu(1)–C(16)–C(17) | 123.5(3) |
| Cu(2)–C(16)–C(17) | 150.0(4) |
| Cu(3)–C(16)–C(17) | 132.8(4) |
| Cu(1)–C(1)–Cu(2) | 74.18(14) |
| Cu(1)–C(1)–Cu(3) | 74.02(13) |
| Cu(2)–C(1)–Cu(3) | 68.39(12) |
| Cu(1)–C(16)–Cu(2) | 70.71(12) |
| Cu(1)–C(16)–Cu(3) | 76.89(13) |
| Cu(2)–C(16)–Cu(3) | 73.33(14) |
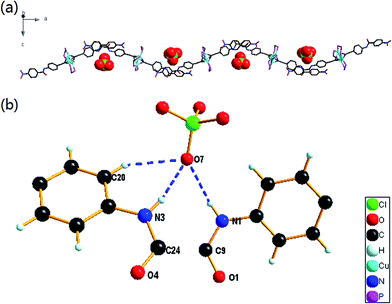
(1) Complexes with NO2 group. In complex 1·PF6, hydrogen bonds are formed between hexafluorophosphate (PF6−) and amide N–Hs as well as hexafluorophosphate anion and aromatic C–Hs in acetylide ligands (Fig. 2(b)). Hexafluorophosphate anion in 1·PF6 is five-coordinated. Each hexafluorophosphate anion is surrounded by two amide clefts of two trinuclear copper(I) complexes and coordinated mainly by two hydrogen bonds from amide groups, which were supplemented by three additional C–H⋯F interactions. The hydrogen bond distances (N⋯F) and angles of N–H⋯F in 1·PF6 are in the range of 3.1336–3.2179 Å and 142–154°, respectively (Table 2). The supporting interactions C–H⋯F are weaker than N–H⋯F with longer bond distances (C⋯F, 3.3152–3.3478 Å) and similar C–H⋯F angles (146–155°). The dihedral angle of adjacent Cu3 plane (plane Cu(1)–Cu(2)–Cu(3) and plane Cu(1′)–Cu(2′)–Cu(3′)) is 51.16°. Two adjacent “Cu3 cluster ligands” around hexafluorophosphate anion are in a bended arrangement, with an dihedral angle between plane N(1)–C(9)–O(1) and plane N(3′)–C(24′)–O(4′) being 25.20°. As a ditopic anion binding ligand, each cation 1 binds two PF6− simultaneously. Therefore, complex 1·PF6 shows an infinite one-dimensional structure, which can be viewed as anion coordination polymers, or ACPs, in which the hexafluorophosphate anions function as the coordination nodes like the metal ions in CPs. In this polymeric structure, the anions are regularly arranged in an almost linear array, and a 1D infinite zigzag hydrogen bonding polymeric chain are formed by the bended cation 1 together with bridged PF6− (Fig. 2(a)). The fluorine atoms F(3) in PF6− which is used to bind with amide group are regularly arranged in an almost linear array, wherein the distance between two adjacent F atoms and the F(3)⋯F(3)⋯F(3) angle are 18.79 Å and 172.54°, respectively.
| Complexes | D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | ∠(DHA) | Symmetry code |
|---|---|---|---|---|---|---|
| 1·BF4 | N(120)–H(12B)⋯F(1) | 0.86 | 2.28 | 2.9773 | 138 | |
| N(122)–H(12A)⋯F(1) | 0.86 | 2.49 | 3.2826 | 153 | 1 − x, 1/2 + y, 1/2 − z | |
| C(89)–H(89A)⋯F(1) | 0.93 | 2.49 | 3.3395 | 152 | ||
| 1·PF6 | N(1)–H(1)⋯F(3) | 0.86 | 2.42 | 3.2179 | 154 | −x, 1/2 + y, 1/2 − z |
| N(3)–H(3)⋯F(3) | 0.86 | 2.41 | 3.1336 | 142 | 1/2 − x, 1/2 − y, z | |
| C(7)–H(7)⋯F(2) | 0.93 | 2.48 | 3.3478 | 155 | −x, 1/2 + y, 1/2 − z | |
| C(7)–H(7)⋯F(3) | 0.93 | 2.50 | 3.3259 | 147 | −x, 1/2 + y, 1/2 − z | |
| C(20)–H(20)⋯F(5) | 0.93 | 2.50 | 3.3152 | 146 | 1/2 − x, 1/2 − y, z | |
| 1·ClO4 | N(1)–H(1)⋯O(7) | 0.88 | 2.36 | 3.075(9) | 138 | 3/2 − x, 1/2 − y, z |
| N(3)–H(3)⋯O(7) | 0.88 | 2.47 | 3.291(11) | 155 | 2 − x, 1/2 + y, 1/2 − z | |
| C(20)–H(20)⋯O(7) | 0.95 | 2.47 | 3.342(12) | 152 | 2 − x, 1/2 + y, 1/2 − z | |
| 2·BF4 | N(1)–H(1)⋯O(2) | 0.86 | 2.18 | 2.852(3) | 135 | 1/2 − x, −1/2 + y, 1/2 − z |
| C(56)–H(56)⋯F(1) | 0.93 | 2.54 | 3.459(5) | 169 | x, 1 + y, z | |
| C(65)–H(65)⋯F(2) | 0.93 | 2.54 | 3.300(4) | 139 | 1/2 − x, 1/2 + y, 1/2 − z | |
| 3·BF4 | N(1)–H(1)⋯O(2) | 0.86 | 2.16 | 2.957(6) | 154 | x, −y, 1/2 + z |
| C(5)–H(5)⋯O(2) | 0.93 | 2.48 | 3.256(6) | 142 | x, −y, 1/2 + z | |
| C(11)–H(11)⋯O(2) | 0.93 | 2.47 | 3.333(9) | 155 | x, −y, 1/2 + z | |
| N(2)–H(2)⋯F(13) | 0.86 | 2.13 | 2.870(6) | 144 | ||
| C(20)–H(20)⋯F(14) | 0.93 | 2.51 | 3.381(7) | 156 | ||
| 4·BF4 | N(2)–H(2)⋯F(2) | 0.88 | 2.23 | 3.077(6) | 162 | x, 1/2 − y, 1/2 + z |
| C(21)–H(21)⋯F(2) | 0.95 | 2.51 | 3.326(7) | 144 | x, 1/2 − y, 1/2 + z | |
| C(27)–H(27)⋯F(2) | 0.95 | 2.41 | 3.343(8) | 166 | x, 1/2 − y, 1/2 + z | |
| 4·F | N(2)–H(2A)⋯O(1) | 0.88 | 2.48 | 3.244(4) | 146 | 1 + x, y, z |
| C(27)–H(27A)⋯O(1) | 0.95 | 2.59 | 3.527(4) | 168 | 1 + x, y, z | |
| N(1)–H(1A)⋯F(1) | 0.88 | 1.95 | 2.811(5) | 165 | −1/2 + x, 3/2 − y, 1/2 + z | |
| C(7)–H(7A)⋯F(1) | 0.95 | 2.40 | 3.189(5) | 140 | −1/2 + x, 3/2 − y, 1/2 + z | |
| C(15)–H(15A)⋯F(1) | 0.95 | 2.48 | 3.140(5) | 126 | −1/2 + x, 3/2 − y, 1/2 + z |
Complexes 1·BF4 and 1·ClO4 adopt a similar structure with that of 1·PF6 owing to the same substituent group NO2 they have. The hydrogen bond distances and angles in 1·BF4 and 1·ClO4 are listed in Table 2. Each tetrafluoroborate or perchlorate anion is surrounded by two amide clefts of two trinuclear copper(I) complexes and coordinated mainly by two hydrogen bonds from amide groups, which were supplemented by additional C–H⋯F or C–H⋯O interactions (Fig. 3(b) and 4(b)). However, their configurations are affected by the counter anions with varied size, shape and basicity. In contrast to octahedral PF6−, BF4− and ClO4− with tetrahedral geometry in this system are three-coordinated. Only one atom in each anion is able to form hydrogen bond with two ligands in adjacent cations 1. The hydrogen bond distances (N⋯F) and angles of N–H⋯F in 1·BF4 are in the range of 2.9773–3.2826 Å and 138–153°, respectively (Table 2). While the hydrogen bond distances (N⋯O) and angles of N–H⋯O in 1·ClO4 are in the range of 3.075(9)–3.291(11) Å and 138–155°, respectively (Table 2). The configuration of 1·BF4 is supplemented by an additional C–H⋯F bond (C⋯F distance 3.3395 Å; C–H⋯F angle 152°), and 1·ClO4 by a C–H⋯O bond (C⋯O distance 3.342(12) Å; C–H⋯O angle 152°). The stronger basicity of BF4− results in the shorter hydrogen bond distances in 1·BF4 when compared with their counterparts in 1·ClO4. The dihedral angle of adjacent Cu3 plane (plane Cu(1)–Cu(2)–Cu(3) and plane Cu(1′)–Cu(2′)–Cu(3′)) is 46.89° for 1·BF4, and 47.59° for 1·ClO4. Similar to the two unparalleled ligands in 1·PF6, the dihedral angle of plane N(120)–C(107)–O(117) and plane N(122′)–C(91′)–O(114′) is 26.02° in 1·BF4, and that of plane N(1)–C(9)–O(1) and plane N(3′)–C(24′)–O(4′) is 26.73° in 1·ClO4. Thus, ACPs 1·BF4 and 1·ClO4 adopt a 1D infinite zigzag structure as that of 1·PF6, with tetrahedral BF4− or ClO4− as node and bended cations 1 as ligand (Fig. 3(a) and 4(a)). The binding atoms F(1) in ACP 1·BF4 and O(7) in ACP 1·ClO4 are almost aligned (F(1)⋯F(1)⋯F(1) angle 175.32° and O(7)⋯O(7)⋯O(7) angle 176.03°), with the distances between two binding atoms are 18.79 Å (1·BF4) and 18.81 Å (1·ClO4), respectively.
(2) Complexes with CF3 group. Infinite arrangement can also be found in complex 2·BF4, while in a different way from complexes with nitro group, owing to the differences in their shapes as well as electron-withdrawing properties. In 2·BF4, hydrogen bonds are formed between two adjacent amide N–Hs as well as tetrafluoroborate anions and aromatic C–Hs in dppm ligands (Fig. 5(b)). The hydrogen bond distances and angles in 2·BF4 are listed in Table 2. Two amide groups in adjacent complexes form the N–H⋯O hydrogen bond, which is the basic interaction in this system to maintain the polymeric structure. The two amide groups are in almost right-angle bended arrangement, with the dihedral angle of plane N(1)–C(18)–O(1) and plane N(2′)–C(92′)–O(2′) being 80.94°. The N–H⋯O hydrogen bonds with N⋯O distance at 2.8566 Å and angle at 135°, respectively, are supplemented by two additional C–H⋯F interactions between dppm ligands and tetrafluoroborate anion. Tetrafluoroborate anions in 2·BF4 are two-coordinated. The hydrogen bond distances (C⋯F) and angles of C–H⋯F in 2·BF4 are in the range of 3.300(4)–3.459(5) Å and 139–169°, respectively (Table 2). The dihedral angle of adjacent Cu3 planes (plane Cu(1)–Cu(2)–Cu(3) and plane Cu(1′)–Cu(2′)–Cu(3′)) is 71.35°, which is in accord with the right-angle arrangement of amide groups. The dihedral angle between two benzene rings used to bind anions is 72.32°. In addition, the BF4− anions are regularly arranged in an almost right-angle array, wherein the distance between two boron atoms and the B(1)⋯B(1)⋯B(1) angle are 16.03 Å and 88.17°, respectively. Thus, complex 2·BF4 can be regarded as a zigzag 1D ACP formed between cations 2 and bridged BF4− (Fig. 5(a)).
(3) Complexes without substituent group. Different from complexes with electron-withdrawing groups, the cation 3 in complex 3·BF4 could not bind two anions at the same time, and thus ACPs could not be observed in this system. In complex 3·BF4, anions BF4− are two-coordinated (Fig. 6(b)). A N–H⋯F hydrogen bond (N⋯F distance 2.870(6) Å; N–H⋯F angle 144°) and an additional C–H⋯F bond (C⋯F distance 3.381(7) Å; C–H⋯F angle 156°) are used to coordinate one of the amide groups in cation 3 (Table 2). In addition, two adjacent cations are interconnected via N(1)–H(1)⋯O(2) with N⋯O distance at 2.957(6) Å and angle at 154°, respectively, and two supporting interactions C(5)–H(5)⋯O(2) and C(11)–H(11)⋯O(2) (the C⋯O distances and angles range from 3.256(6)–3.333(9) Å and 144–155°, respectively), which results in an infinite construction (Table 2). The dihedral angle of plane N(1)–C(9)–O(1) and plane N(2′)–C(24′)–O(2′) is 74.12° and the dihedral angle of adjacent Cu3 planes (plane Cu(1)–Cu(2)–Cu(3) and plane Cu(1′)–Cu(2′)–Cu(3′)) is 86.63°, which is in accord with the right-angle arrangement of amide planes. As a ditopic ligand, cations 3 form a 1D infinite meso-helical hydrogen bonding polymeric chain with a counter anion bound in each cation (Fig. 6(a)). The BF4− anions are regularly arranged in an almost right-angle array, wherein the distance between two boron atoms and the B(4)⋯B(4)⋯B(4) angle are 20.84 Å and 72.17°, respectively. The meso-helical chain in complex 3·BF4 has a pitch length of 24.55 Å.
 | ||
| Fig. 6 The crystal structure of 3·BF4, (a) 1D polymeric chain; (b) anion coordination environment in ACPs. | ||
(4) Complexes with OCH3 group. In complex 4·BF4, one of the amide group in cation 4 coordinates the counter anion BF4− via a N–H⋯F hydrogen bond, which is supplemented by two additional C–H⋯F bonds (Fig. 7). Tetrafluoroborate anions in 4·BF4 are three-coordinated. The hydrogen bond distance and angle of N–H⋯F in 4·BF4 are 3.077(6) Å and 162°, and those of C–H⋯F bonds are in the range of 3.326(7)–3.343(8) Å and 144–166°, respectively (Table 2). However, different from complexes with electron-withdrawing group, 4·BF4 is unable to bind two anions simultaneously, therefore cannot construct stable polymeric chain using anions as nodes.
Complex 4·F was obtained upon addition of excess fluoride anions into the solution of 4·BF4. Its structure is similar to complex 4·BF4, consisting of a bended cation 4 and a fluoride anion bound at one side by amide group (Fig. 8(b)). Hydrogen bonds involve fluoride anions in this system include N(1)–H(1A)⋯F(1) (N⋯F distance 2.805(3) Å; N–H⋯F angle 165°) and two additional C–H⋯F bonds (C⋯F distance 3.141(4)–3.189(4) Å; N–H⋯F angle 126–140°) (Table 2). Due to the stronger basicity and smaller size of fluoride anion, the average hydrogen bond distance in 4·F is shorter than 4·BF4 considerably. Furthermore, resemble complex 3·BF4, two cations in 4·F are held together by N(2)–H(2A)⋯O(1) (N⋯O distance 3.241(3) Å; N–H⋯O angle 146°), and supported by C(27)–H(27A)⋯O(1) (C⋯O distance 3.522(3) Å; C–H⋯O angle 168°). The dihedral angle of plane N(1)–C(9)–O(1) and plane N(2′)–C(25′)–O(3′) is 54.87°. Two adjacent Cu3 planes (plane Cu(1)–Cu(2)–Cu(3) and plane Cu(1′)–Cu(2′)–Cu(3′)) are nearly parallel, with a dihedral angle being 0° and the identity distance being 15.94 Å. In the meanwhile, the anions are regularly arranged in an almost linear array, wherein the distance between two fluoride anions and the F(1)⋯F(1)⋯F(1) angle are 17.91 Å and 180.00°, respectively. Therefore, cations 4 together with F− form the 1D infinite linear hydrogen bonding polymeric chains (Fig. 8(a)).
 | ||
| Fig. 8 The crystal structure of 4·F, (a) 1D polymeric chain; (b) anion coordination environment in polymeric chain. | ||
(5) Structural diversity of complexes. Seven complexes reported in this work bear five kinds of architectures (Fig. 9). In complexes with nitro group 1·BF4, 1·PF6, and 1·ClO4, each anion is bound to two ligands from adjacent complexes at amide sites. In other words, 1D zigzag polymeric chains are formed using anions as node and complexes as cations. In this case, difference in anions results merely in different angles, rather than diverse architectures. When compared with nitro-substituted complexes, complexes with less electron-withdrawing group 2·BF4 are unable to bind anions by their amide groups, due to the less acidity of amide N–Hs here. In 2·BF4, zigzag polymeric chains are formed via hydrogen bonds between two amide groups in adjacent complexes and supported by interactions between dppm ligands and tetrafluoroborate anion. While in complex without substituent group 3·BF4, 1D ACP could not be observed owing to the absence of the electron-withdrawing group. However, adjacent cations are interconnected to construct a 1D infinite meso-helical hydrogen bonding polymeric chain with a counter anion bound in each cation. As for complexes with electron-donating group, 4·BF4 and 4·F, structures varied dramatically with different anions. Owing to the weak hydrogen bond donor in 4·BF4, polymeric structure could not be found. However, with stronger base F−, infinite linear chains are formed by amide groups' hydrogen bonds and anions are bound to one of the amide groups. As what we can see, assembly of various anions and cations with different substituent groups results in the diversity of trinuclear copper(I) acetylide complexes.
Electronic absorption and emission spectra of complexes 1·BF4–4·BF4
The photophysical data for complexes 1·BF4–4·BF4, 1·PF6, 1·ClO4 and 4·F are summarized in Table 3. For comparison, the photophysics of acetylide ligands L1–L4 are studied and listed in Table S7 (ESI†), as well. The electronic absorption spectrum of 1·BF4 in DMSO at 298 K (Fig. S2, ESI†) shows a high-energy band at ca. 266 and a shoulder at ca. 302 nm, which are assigned to ligand-centred π → π* (dppm) and π → π* (acetylide) transitions, respectively, owing to the similar absorption energies with those of the free dppm ligand and acetylenes. The lower energy absorption shoulder at ca. 336 nm is probably the charge transfer transition from the amide to the NO2 group in the acetylide ligand. The electronic absorption spectra of complexes 1·PF6 and 1·ClO4 are similar to that of 1·BF4 (Fig. S2, ESI†), indicating that counter anions exert slight effect on absorption intensity and wavelength. The electronic absorption spectra of non-nitro derivatives 2·BF4–4·BF4 in DMSO at 298 K (Fig. S3, ESI†) exhibit two absorption bands at ca. 268 and 345–350 nm. The band at ca. 268 nm is ascribed to ligand-centred π → π* (dppm) transition, while low-energy bands at 345–350 nm are assigned as the admixture of metal-perturbed ligand-centered π–π* (acetylide) and LMCT (acetylide → Cu3) transition.44–45 4·F shows similar electronic absorption spectrum with 4·BF4, expect for the slight decrease in the molar absorption coefficient (Fig. S4, ESI†).| Complexes | Medium | λabs/nm (ε/dm3 mol−1 cm−1) | λem/nm (εem/τs) | Φem |
|---|---|---|---|---|
| 1·BF4 | DMSO | 266 (83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540), 302 (sh, 60 540), 302 (sh, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910), 336 (sh, 45 910), 336 (sh, 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460) 460) |
474 (0.1) | 0.014 |
| Solid | 505 (7.3) | |||
| 1·PF6 | DMSO | 266 (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860), 302 (sh, 63 860), 302 (sh, 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030), 336 (sh, 46 030), 336 (sh, 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570) 570) |
474 (0.1) | 0.016 |
| Solid | 504 (max, 6.7), 544 (sh) | |||
| 1·ClO4 | DMSO | 266 (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910), 302 (sh, 63 910), 302 (sh, 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430), 336 (sh, 44 430), 336 (sh, 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440) 440) |
474 (0.1) | 0.015 |
| Solid | 506 (11.1) | |||
| 2·BF4 | DMSO | 268 (58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990), 350 (44 990), 350 (44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950) 950) |
475 (0.1) | 0.051 |
| Solid | 507 (14.7) | |||
| 3·BF4 | DMSO | 268 (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810), 346 (45 810), 346 (45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760) 760) |
502 (0.3) | 0.078 |
| Solid | 514 (max, 75.3), 557 (sh) | |||
| 4·BF4 | DMSO | 268 (58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180), 345 (47 180), 345 (47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880) 880) |
500 (0.3) | 0.086 |
| Solid | 514 (max, 21.9), 557 (sh) | |||
| 4·F | DMSO | 268 (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660), 345 (46 660), 345 (46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 970) 970) |
499 (0.3) | 0.078 |
| Solid | 513 (25.1) |
Excitation at λ > 370 nm of complexes 1·BF4–4·BF4, 1·PF6, 1·ClO4 and 4·F in the solid state and in DMSO solution results in long-lived and intense luminescence in the visible light regime at 298 K, with emission quantum yields of 1.4 × 10−2 to 8.6 × 10−2 in DMSO solutions. Fig. 10 displays the emission spectrum of 3·BF4 in the solid state at 298 K, in which a broad band at ca. 514 nm and a shoulder at ca. 557 nm are observed. The spacing of the adjacent band 3·BF4 is ca. 1500 cm−1, which is typical of ground-state aromatic ν(C⋯C) stretching frequency.45 The solid state emission spectra of 1·BF4, 2·BF4, 4·BF4, 1·PF6, 1·ClO4 and 4·F (Fig. S5–10, ESI†) are similar to that of 3·BF4 with lifetimes in microsecond range, which is suggestive of the involvement of a spin-forbidden transition. In general, the complexes with electron-rich acetylides emit at a lower energy. The electron-donating substituent R would increase the energy of the π orbital of the acetylides and thus decrease the energy of the LMCT excited state. Therefore, the origin of the emission is proposed to involve substantial 3LMCT [acetylide → Cu3] character.44–45 In DMSO solution, trinuclear copper(I) acetylide complexes 1·BF4–4·BF4, 1·PF6, 1·ClO4 and 4·F exhibit blue-green to yellow-green emission at 298 K (Fig. S11–17, ESI†). A broad band at ca. 475–500 nm is observed, which follows the same trend with that in solid state. Thus, the emission in DMSO solution is ascribed to LMCT as well.44–45 For the trinuclear copper(I) acetylide complexes studied in this paper, the emission energies depend mainly on the substituent R on the acetylide ligand, while the type of counter anions have little effect on the electronic absorption as well as emission spectra both in solid state and DMSO solution.
Anion binding properties of complexes 1·BF4–4·BF4
The anion-binding properties of complexes 1·BF4–4·BF4 have been investigated by 1H NMR spectroscopy. The results of 1H NMR titration studies with a variety of anions show that even in a competitive solvent (DMSO-d6) interactions and selectivity could still be observed. All of the anions used were in the form of tetra-n-butylammonium salts. Unfortunately, due to the decomposition of the trinuclear complexes upon addition of NBu4H2PO4 and NBu4HSO4, the investigations toward H2PO4− and HSO4− were not carried out.Fig. S18 (ESI†) shows the 1H NMR spectral changes of 1·BF4 upon addition of Cl− in DMSO-d6. Upon the addition of chloride anion, the signals of the N–H protons (Ha) show a relatively considerable downfield shift, while the other proton signals are found to undergo essentially negligible changes, which suggests the formation of a hydrogen bonding interaction between the amide groups in 1·BF4 and Cl−. The slight downfield shift of protons Hc and Hd on the phenyl ring is ascribed to the polarization effect of the C–H bond that is introduced by the through-space effect.62–64 Analogous investigations have also been carried out with Y-shape anion OAc− and larger halides Br− and I− (Fig. S19–21, ESI†). The magnitude of the complexation-induced 1H NMR shift upon addition of OAc− is larger when compared with that of Cl−, while the signal of the N–H protons (Ha) shows slight change with Br−, and none when I− was added. For other complexes, 2·BF4–4·BF4, the anion binding properties were also studied (Fig. 11 and S22, ESI†), which show similar binding trend with 1·BF4, but weaker binding ability.
 | ||
| Fig. 11 The shifts of the signals of amide N–H (Ha) of complexes (a) 1·BF4, (b) 2·BF4, (c) 3·BF4, and (d) 4·BF4 upon addition of different anions with different concentrations in DMSO-d6 at 298 K. | ||
Unfortunately, we were unable to obtain the anion-binding constants of complexes 1·BF4–4·BF4 by nonlinear least-square fits of the shifts of the signals of amide N–H (Ha) versus the concentration of the added anions, owing to the small changes in the amide N–H (Ha) chemical shifts. As a result, we could only compare the signal changes upon addition of different anions in the same amount. In general, the signal changes of different complexes with the same anion are in the following order: R = NO2 (1·BF4) > CF3 (2·BF4) > H (3·BF4) > OCH3 (4·BF4), which is in line with the decreasing of the electron-withdrawing ability of substituent R on the acetylide ligands (Fig. S22, ESI†). This could be rationalized by the fact that the stronger electron-withdrawing substituent R on the acetylide ligand could induce higher acidity of amide group, which strengthen the hydrogen bond interactions between complexes and anions. The signal changes of the same complex with the various anions are in the following order: OAc− > Cl− > Br− > I−, which is in line with the decreasing of the basicity of anions (Fig. 11).
The interactions of 1·BF4–4·BF4 with F− were investigated and exhibited different spectral changes from other anions. Fig. S23 (ESI†) shows the 1H NMR spectral changes of 1·BF4 in DMSO-d6 upon addition of F−. The significant downfield shift of the signal of amide N–H (Ha) is observed upon addition of F− from 0 to 1 equiv., while this peak disappear rapidly when the amount of F− added was larger than 1 equiv., and the aromatic proton signals Hb and Hd showed a slight upfield shift, which could be ascribed to the deshielding effect resulting from the increased electron density of the phenyl ring,65 induced by the deprotonation of the amide N–H unit. During the addition of F−, the color of the solution of 1·BF4 in DMSO-d6 changes from orange to dark red. After the addition of 3 equiv. of F−, a distinct triplet centered at 16.08 ppm (J = 120 Hz) appears, which is assigned as the formation of HF2−.66–67 In addition, its 19F NMR spectrum also displays a distinct doublet centered at −143.13 ppm (J = 117 Hz) (Fig. S24, ESI†), suggesting the formation of HF2−.66–67 These results indicate the deprotonation of the amide N–H of 1·BF4 upon addition of F− in DMSO-d6. Complexes 1·PF6, 1·ClO4 and 2·BF4–4·BF4 show similar color and spectral changes upon addition of F−, which could be ascribed to deprotonation as well (Fig. S25–S34, ESI†).
We have also examined the color change of complexes 1·BF4 with different anions in DMSO (Fig. S35 and S36, ESI†). No color change of 1·BF4 in DMSO could be observed upon addition of anions, except F−. Thus, 1·BF4 shows selective color change towards F− in DMSO. Complexes 2·BF4–4·BF4 exhibited similar selectivity through dramatic color change, which allows F− detection with naked eyes. Even though addition of the anions studied in this paper into the solutions of 1·BF4–4·BF4 did cause their UV-Vis spectral changes, the changes were too small to compare the binding abilities of complexes 1·BF4–4·BF4 towards anions.
Conclusions
In summary, a series of discrete or polymeric amide based trinuclear copper(I) complexes 1·BF4–4·BF4, 1·PF6, 1·ClO4 and 4·F have been synthesized and characterized, with their crystal structures determined. Among them, 1D hydrogen bonding polymeric chain with zigzag, meso-helical or linear structures are observed. The architectures of these complexes could be perturbed by anions and the R groups on “Cu3 cluster ligands”. Complexes 1·BF4–4·BF4, 1·PF6, 1·ClO4 and 4·F exhibit luminescence both in the solid state and in the DMSO solution at 298 K. The anion binding abilities of complexes 1·BF4–4·BF4 toward different anions have also been studied by NMR and UV-vis. Their selective color change upon addition of F− enables the naked eye detection of F−.Experimental section
Materials and reagents
Dinuclear complexes [Cu2(μ-dppm)2(CH3CN)4](BF4)2, [Cu2(μ-dppm)2(CH3CN)4](PF6)2, and [Cu2(μ-dppm)2(CH3CN)4](ClO4)2 were synthesized according to literature procedures.68 Bis(diphenylphosphino)methane (dppm) and benzoyl chloride were purchased from Alfa-Aesar. 4-Ethylnylaniline and tetra-n-butylammonium iodide were purchased from Acros. 4-Nitrobenzoyl chloride was purchased from TCI. 4-Methoxybenzoyl, 4-trifluoromethylbenzoyl chloride and tetra-n-butylammonium bromide hydrate were obtained from J&K. Tetra-n-butylammonium fluoride hydrate and tetra-n-butylammonium acetate was obtained from Sigma-Aldrich. All reactions were carried out under anhydrous and anaerobic conditions using standard Schlenk techniques under nitrogen. All solvents were purified and distilled using standard procedures before use. All other reagents were of analytical grade and were used as received.Physical measurements and instrumentation
Chemical shifts (δ, ppm) were reported relative to tetramethylsilane for 1H NMR, and NaF for 19F NMR on a Varian Mercury-Plus 300 spectrometer, 85% H3PO4 for 31P NMR on a Bruker Avance III 400 MHz spectrometer. Emission spectra were obtained on a FLS980 fluorescence spectrophotometer. The solution emission quantum yields were measured using quinine sulfate in 1.0 N sulfuric acid as standard69 (Φr = 0.546, excitation wavelength at 365 nm) and calculated by Φem = Φr(Br/Bs)(ns/nr)2(Ds/Dr), where the subscripts s and r refer to sample and reference standard solution respectively, n is the refractive index of the solvents, D is the integrated intensity, and Φ is the luminescence quantum yield. The quantity B is calculated by B = 1 − 10−AL, where A is the absorbance at the excitation wavelength and L is the optical path length. Infra-red spectra were recorded from KBr pellets in the range of 400–4000 cm−1 on a Bruker-EQUINOX 55 FT-IR spectrometer. Electrospray ionization (ESI) mass spectra were recorded on a LCQ DECA XP quadrupole ion trap mass spectrometer and mass spectra of ligands L1–L4 and complexes 1·BF4–4·BF4, 1·PF6, 1·ClO4 and 4·F are listed in Fig. S37 and S38 (ESI†), respectively. Elemental analysis was performed on an Elemental Vario EL elemental analyzer.Crystal structure determination
Crystals were grown by diffusion of diethyl ether into concentrated solution of the corresponding complexes. Single crystals of 1·BF4–4·BF4, 1·PF6, 1·ClO4 and 4·F were carefully picked and coated in paratone oil, attached to a glass silk inserted in a stainless steel stick, then quickly transferred to the Agilent Gemini S Ultra CCD Diffractometer with the enhance X-ray source of Cu radiation (λ = 1.54178 Å) using the ω–ϕ scan technique. Structural solution and refinement against F2 were carried out using the SHELXL programs.70 Hydrogen atoms were placed in geometrically calculated positions and included in the refinement process using riding model with isotropic thermal parameters: Uiso(H) = 1.2Ueq(−CH). For structures of 1·BF4–4·BF4, 1·ClO4 and 4·F, the contribution of heavily disordered solvent molecules was treated by the Squeeze procedure implemented in Platon.71–72 Crystallographic data for the structures reported in this paper have been deposited in the Cambridge Crystallographic Data Centre as supplementary publication, CCDC 1421592–1421598 for 1·BF4, 1·ClO4, 1·PF6, 2·BF4–4·BF4 and 4·F.†Titrations
For a typical 1H NMR titration experiment, 1 μL aliquots of a tetra-n-butylammonium salt (5.00 × 10−1 mol dm−3 in DMSO-d6) were added into the 0.5 mL solution of the copper(I) acetylide complex in DMSO-d6 (5.00 × 10−3 mol dm−3) by a syringe, and the 1H NMR spectral changes were recorded by a Varian Mercury-Plus 300 spectrometer at 298 K.Synthesis
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) CC6H4-4-NHC(O)-C6H4-R (R = NO2 (L1), CF3 (L2), H (L3), OCH3 (L4)). To a solution of 4-ethylnylaniline and 1 equiv. of the corresponding acyl chloride in CHCl3 was added triethylamine. The mixture was heated to reflux for 18 h. The solvent was removed under reduced pressure, and the residue was washed with water and n-hexane to yield pale yellow solid.
CC6H4-4-NHC(O)-C6H4-R (R = NO2 (L1), CF3 (L2), H (L3), OCH3 (L4)). To a solution of 4-ethylnylaniline and 1 equiv. of the corresponding acyl chloride in CHCl3 was added triethylamine. The mixture was heated to reflux for 18 h. The solvent was removed under reduced pressure, and the residue was washed with water and n-hexane to yield pale yellow solid.
L1. Yield: 123.5 mg, 56%. 1H NMR (DMSO-d6, 298 K): δ = 10.71 (s, 1H, NH), 8.36 (d, 2H, J = 9 Hz, aromatic ring), 8.16 (d, 2H, J = 9 Hz, aromatic ring), 7.80 (d, 2H, J = 9 Hz, aromatic ring), 7.48 (d, 2H, J = 9 Hz, aromatic ring), 4.15 (s, 1H, HC
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C). IR (KBr, cm−1): ν = 3255 (N–H), 2098 (C
C). IR (KBr, cm−1): ν = 3255 (N–H), 2098 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1654 (C
C), 1654 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS: m/z = 265 [M − H]−. Anal. calcd for C15H10N2O3 (%): C, 67.67; H, 3.79; N, 10.52. Found: C, 67.41; H, 3.80; N, 10.48.
O). ESI-MS: m/z = 265 [M − H]−. Anal. calcd for C15H10N2O3 (%): C, 67.67; H, 3.79; N, 10.52. Found: C, 67.41; H, 3.80; N, 10.48.
L2. Yield: 139.8 mg, 60%, 1H NMR (DMSO-d6, 298 K): δ = 10.60 (s, 1H, NH), 8.12 (d, 2H, J = 9 Hz, aromatic ring), 7.91 (d, 2H, J = 8 Hz, aromatic ring), 7.80 (d, 2H, J = 9 Hz, aromatic ring), 7.47 (d, 2H, J = 9 Hz, aromatic ring), 4.14 (s, 1H, HC
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C). IR (KBr, cm−1): ν = 3302 (N–H), 2116 (C
C). IR (KBr, cm−1): ν = 3302 (N–H), 2116 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1657 (C
C), 1657 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS: m/z = 288 [M − H]−. Anal. calcd for C16H10F3NO (%): C, 66.44; H, 3.48; N, 4.84. Found: C, 66.40; H, 3.47; N, 4.83.
O). ESI-MS: m/z = 288 [M − H]−. Anal. calcd for C16H10F3NO (%): C, 66.44; H, 3.48; N, 4.84. Found: C, 66.40; H, 3.47; N, 4.83.
L3. Yield: 115.2 mg, 56%, 1H NMR (DMSO-d6, 298 K): δ = 10.40 (s, 1H, NH), 7.92 (d, 2H, J = 8 Hz, aromatic ring), 7.80 (d, 2H, J = 9 Hz, aromatic ring), 7.61–7.44 (m, 5H, aromatic ring), 4.12 (s, 1H, HC
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C). IR (KBr, cm−1): ν = 3299 (N–H), 2106 (C
C). IR (KBr, cm−1): ν = 3299 (N–H), 2106 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1659 (C
C), 1659 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS: m/z = 256 [M − H]−. Anal. calcd for C15H11NO (%): C, 81.43; H, 5.01; N, 6.33. Found: C, 80.41; H, 5.01; N, 6.35.
O). ESI-MS: m/z = 256 [M − H]−. Anal. calcd for C15H11NO (%): C, 81.43; H, 5.01; N, 6.33. Found: C, 80.41; H, 5.01; N, 6.35.
L4. Yield: 174.2 mg, 59%, 1H NMR (DMSO-d6, 298 K): δ = 10.22 (s, 1H, NH), 7.93 (d, 2H, J = 9 Hz, aromatic ring), 7.79 (d, 2H, J = 8 Hz, aromatic ring), 7.43 (d, 2H, J = 9 Hz, aromatic ring), 7.05 (d, 2H, J = 9 Hz, aromatic ring), 4.10 (s, 1H, HC
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 3.83 (s, 3H, OCH3). IR (KBr, cm−1): ν = 3283 (N–H), 2106 (C
C), 3.83 (s, 3H, OCH3). IR (KBr, cm−1): ν = 3283 (N–H), 2106 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1658 (C
C), 1658 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS: m/z = 250 [M − H]−. Anal. calcd for C16H13NO2 (%): C, 76.48; H, 5.21; N, 5.57. Found: C, 76.50; H, 5.22; N, 5.55.
O). ESI-MS: m/z = 250 [M − H]−. Anal. calcd for C16H13NO2 (%): C, 76.48; H, 5.21; N, 5.57. Found: C, 76.50; H, 5.22; N, 5.55.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) CC6H4-4-NHC(O)C6H4-4-NO2)2BF4]∞ (1·BF4). To a solution of [Cu2(μ-dppm)2(CH3CN)4](BF4)2 (100.8 mg, 0.082 mmol) and L1 (29.0 mg, 0.11 mmol) in degassed CH3CN (50 mL), NEt3 (1 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated CH3CN solution gave orange crystals. Yield: 90.6 mg, 85%.1H NMR (CD3CN, 298 K): δ = 9.19 (s, 2H, NH), 8.40 (d, 4H, J = 9 Hz, aromatic ring), 8.21 (d, 4H, J = 9 Hz, aromatic ring), 7.90 (d, 4H, J = 9 Hz, aromatic ring), 7.47 (d, 4H, J = 9 Hz, aromatic ring), 7.18–6.83 (m, 60H, aromatic ring), 3.37 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −5.96 (s). 19F NMR (CD3CN, 298 K): δ = −151.65 (s, 10BF4−), −151.70 (s, 11BF4−). IR (KBr, cm−1): ν = 3373 (N–H), 2138 (C
CC6H4-4-NHC(O)C6H4-4-NO2)2BF4]∞ (1·BF4). To a solution of [Cu2(μ-dppm)2(CH3CN)4](BF4)2 (100.8 mg, 0.082 mmol) and L1 (29.0 mg, 0.11 mmol) in degassed CH3CN (50 mL), NEt3 (1 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated CH3CN solution gave orange crystals. Yield: 90.6 mg, 85%.1H NMR (CD3CN, 298 K): δ = 9.19 (s, 2H, NH), 8.40 (d, 4H, J = 9 Hz, aromatic ring), 8.21 (d, 4H, J = 9 Hz, aromatic ring), 7.90 (d, 4H, J = 9 Hz, aromatic ring), 7.47 (d, 4H, J = 9 Hz, aromatic ring), 7.18–6.83 (m, 60H, aromatic ring), 3.37 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −5.96 (s). 19F NMR (CD3CN, 298 K): δ = −151.65 (s, 10BF4−), −151.70 (s, 11BF4−). IR (KBr, cm−1): ν = 3373 (N–H), 2138 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1672 (C
C), 1672 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS: m/z = 1874 [M]+. Anal. calcd for C105H84Cu3BF4N4O6P6 (%): C, 64.31; H, 4.32; N, 2.86. Found: C, 64.34; H, 4.30; N, 2.87.
O). ESI-MS: m/z = 1874 [M]+. Anal. calcd for C105H84Cu3BF4N4O6P6 (%): C, 64.31; H, 4.32; N, 2.86. Found: C, 64.34; H, 4.30; N, 2.87.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) CC6H4-4-NHC(O)C6H4-4-NO2)2PF6]∞ (1·PF6). To a solution of [Cu2(μ-dppm)2(CH3CN)4](PF6)2 (159.0 mg, 0.11 mmol) and L1 (41.9 mg, 0.16 mmol) in degassed CH3CN (50 mL), NEt3 (1.5 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated CH2Cl2 solution gave orange crystals. Yield: 147.6 mg, 93%. 1H NMR (CD3CN, 298 K): δ = 9.30 (s, 2H, NH), 8.54 (d, 4H, J = 9 Hz, aromatic ring), 8.34 (d, 4H, J = 9 Hz, aromatic ring), 8.04 (d, 4H, J = 9 Hz, aromatic ring), 7.61 (d, 4H, J = 9 Hz, aromatic ring), 7.32–6.97 (m, 60H, aromatic ring), 3.37 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −5.95, −144.65 (quint, PF6−). 19F NMR (CD3CN, 298 K): δ = −73.53 (d, J = 700 Hz). IR (KBr, cm−1): ν = 3399 (N–H), 2321 (C
CC6H4-4-NHC(O)C6H4-4-NO2)2PF6]∞ (1·PF6). To a solution of [Cu2(μ-dppm)2(CH3CN)4](PF6)2 (159.0 mg, 0.11 mmol) and L1 (41.9 mg, 0.16 mmol) in degassed CH3CN (50 mL), NEt3 (1.5 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated CH2Cl2 solution gave orange crystals. Yield: 147.6 mg, 93%. 1H NMR (CD3CN, 298 K): δ = 9.30 (s, 2H, NH), 8.54 (d, 4H, J = 9 Hz, aromatic ring), 8.34 (d, 4H, J = 9 Hz, aromatic ring), 8.04 (d, 4H, J = 9 Hz, aromatic ring), 7.61 (d, 4H, J = 9 Hz, aromatic ring), 7.32–6.97 (m, 60H, aromatic ring), 3.37 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −5.95, −144.65 (quint, PF6−). 19F NMR (CD3CN, 298 K): δ = −73.53 (d, J = 700 Hz). IR (KBr, cm−1): ν = 3399 (N–H), 2321 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1677 (C
C), 1677 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS: m/z = 1874 [M]+. Anal. calcd for C105H84Cu3F6N4O6P7 (%): C, 62.46; H, 4.19; N, 2.77. Found: C, 62.44; H, 4.15; N, 2.72.
O). ESI-MS: m/z = 1874 [M]+. Anal. calcd for C105H84Cu3F6N4O6P7 (%): C, 62.46; H, 4.19; N, 2.77. Found: C, 62.44; H, 4.15; N, 2.72.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) CC6H4-4-NHC(O)C6H4-4-NO2)2ClO4]∞ (1·ClO4). To a solution of [Cu2(μ-dppm)2(CH3CN)4](ClO4)2 (106.7 mg, 0.085 mmol) and L1 (30.3 mg, 0.11 mmol) in degassed CH3CN (50 mL), NEt3 (1 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated CH2Cl2 solution gave orange crystals. Yield: 98.0 mg, 88%.1H NMR (CD3CN, 298 K): δ = 8.98 (s, 2H, NH), 8.21 (d, 4H, J = 9 Hz, aromatic ring), 8.02 (d, 4H, J = 9 Hz, aromatic ring), 7.71 (d, 4H, J = 8 Hz, aromatic ring), 7.28 (d, 4H, J = 9 Hz, aromatic ring), 6.97–6.64 (m, 60H, aromatic ring), 3.04 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −5.95 (s). IR (KBr, cm−1): ν = 3387 (N–H), 2238 (C
CC6H4-4-NHC(O)C6H4-4-NO2)2ClO4]∞ (1·ClO4). To a solution of [Cu2(μ-dppm)2(CH3CN)4](ClO4)2 (106.7 mg, 0.085 mmol) and L1 (30.3 mg, 0.11 mmol) in degassed CH3CN (50 mL), NEt3 (1 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated CH2Cl2 solution gave orange crystals. Yield: 98.0 mg, 88%.1H NMR (CD3CN, 298 K): δ = 8.98 (s, 2H, NH), 8.21 (d, 4H, J = 9 Hz, aromatic ring), 8.02 (d, 4H, J = 9 Hz, aromatic ring), 7.71 (d, 4H, J = 8 Hz, aromatic ring), 7.28 (d, 4H, J = 9 Hz, aromatic ring), 6.97–6.64 (m, 60H, aromatic ring), 3.04 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −5.95 (s). IR (KBr, cm−1): ν = 3387 (N–H), 2238 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1674 (C
C), 1674 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS: m/z = 1874 [M]+. Anal. calcd for C105H84Cu3ClN4O10P6 (%): C, 63.90; H, 4.29; N, 2.84. Found: C, 63.86; H, 4.27; N, 2.86.
O). ESI-MS: m/z = 1874 [M]+. Anal. calcd for C105H84Cu3ClN4O10P6 (%): C, 63.90; H, 4.29; N, 2.84. Found: C, 63.86; H, 4.27; N, 2.86.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) CC6H4-4-NHC(O)C6H4-4-CF3)(μ3-η1-C
CC6H4-4-NHC(O)C6H4-4-CF3)(μ3-η1-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) CC6H4-4-NHC(O)C6H4-4-CF3)BF4]∞ (2·BF4). To a solution of [Cu2(μ-dppm)2(CH3CN)4](BF4)2 (215.1 mg, 0.17 mmol) and L2 (67.3 mg, 0.23 mmol) in degassed CH3CN (50 mL), NEt3 (1 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated CH3CN solution gave colorless crystals. Yield: 148.4 mg, 64%. 1H NMR (CD3CN, 298 K): δ = 9.09 (s, 2H, NH), 8.19 (d, 4H, J = 9 Hz, aromatic ring), 7.91 (d, 4H, J = 9 Hz, aromatic ring), 7.48 (d, 4H, J = 9 Hz, aromatic ring), 7.19–6.84 (m, 64H, aromatic ring), 3.23 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −5.94 (s). 19F NMR (CD3CN, 298 K): δ = −64.06 (s, CF3), −151.71 (s, 10BF4−), −151.77 (s, 11BF4−). IR (KBr, cm−1): ν = 3368 (N–H), 2262 (C
CC6H4-4-NHC(O)C6H4-4-CF3)BF4]∞ (2·BF4). To a solution of [Cu2(μ-dppm)2(CH3CN)4](BF4)2 (215.1 mg, 0.17 mmol) and L2 (67.3 mg, 0.23 mmol) in degassed CH3CN (50 mL), NEt3 (1 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated CH3CN solution gave colorless crystals. Yield: 148.4 mg, 64%. 1H NMR (CD3CN, 298 K): δ = 9.09 (s, 2H, NH), 8.19 (d, 4H, J = 9 Hz, aromatic ring), 7.91 (d, 4H, J = 9 Hz, aromatic ring), 7.48 (d, 4H, J = 9 Hz, aromatic ring), 7.19–6.84 (m, 64H, aromatic ring), 3.23 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −5.94 (s). 19F NMR (CD3CN, 298 K): δ = −64.06 (s, CF3), −151.71 (s, 10BF4−), −151.77 (s, 11BF4−). IR (KBr, cm−1): ν = 3368 (N–H), 2262 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1674 (C
C), 1674 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS: m/z = 1920 [M]+. Anal. calcd for C107H84Cu3BF6N2O2P6 (%): C, 66.92; H, 4.41; N, 1.46. Found: C, 66.82; H, 4.45; N, 1.46.
O). ESI-MS: m/z = 1920 [M]+. Anal. calcd for C107H84Cu3BF6N2O2P6 (%): C, 66.92; H, 4.41; N, 1.46. Found: C, 66.82; H, 4.45; N, 1.46.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) CC6H4-4-NHC(O)C6H5)2BF4]∞ (3·BF4). To a solution of [Cu2(μ-dppm)2(CH3CN)4](BF4)2 (72.4 mg, 0.059 mmol) and L3 (17.3 mg, 0.078 mmol) in degassed CH3CN (50 mL), NEt3 (1 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated CH3OH solution gave colorless crystals. Yield: 50.9 mg, 70%. 1H NMR (CD3CN, 298 K): δ = 8.98 (s, 2H, NH), 8.04 (d, 4H, J = 9 Hz, aromatic ring), 7.92 (d, 4H, J = 9 Hz, aromatic ring), 7.66–7.57 (m, 6H, aromatic ring), 7.48 (d, 4H, J = 9 Hz, aromatic ring), 7.19–6.86 (m, 60H, aromatic ring), 3.25 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −5.98 (s). 19F NMR (CD3CN, 298 K): δ = −151.67 (s, 10BF4−), −151.72 (s, 11BF4−). IR (KBr, cm−1): ν = 3369 (N–H), 2238 (C
CC6H4-4-NHC(O)C6H5)2BF4]∞ (3·BF4). To a solution of [Cu2(μ-dppm)2(CH3CN)4](BF4)2 (72.4 mg, 0.059 mmol) and L3 (17.3 mg, 0.078 mmol) in degassed CH3CN (50 mL), NEt3 (1 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated CH3OH solution gave colorless crystals. Yield: 50.9 mg, 70%. 1H NMR (CD3CN, 298 K): δ = 8.98 (s, 2H, NH), 8.04 (d, 4H, J = 9 Hz, aromatic ring), 7.92 (d, 4H, J = 9 Hz, aromatic ring), 7.66–7.57 (m, 6H, aromatic ring), 7.48 (d, 4H, J = 9 Hz, aromatic ring), 7.19–6.86 (m, 60H, aromatic ring), 3.25 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −5.98 (s). 19F NMR (CD3CN, 298 K): δ = −151.67 (s, 10BF4−), −151.72 (s, 11BF4−). IR (KBr, cm−1): ν = 3369 (N–H), 2238 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1656 (C
C), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS: m/z = 1784 [M]+. Anal. calcd for C107H84Cu3BF6N2O2P6 (%): C, 66.92; H, 4.41; N, 1.46. Found: C, 66.93; H, 4.43; N, 1.43.
O). ESI-MS: m/z = 1784 [M]+. Anal. calcd for C107H84Cu3BF6N2O2P6 (%): C, 66.92; H, 4.41; N, 1.46. Found: C, 66.93; H, 4.43; N, 1.43.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) CC6H4-4-NHC(O)C6H4-4-OCH3)2]BF4 (4·BF4). To a solution of [Cu2(μ-dppm)2(CH3CN)4](BF4)2 (114.8 mg, 0.093 mmol) and L4 (31.6 mg, 0.13 mmol) in degassed CH3CN (50 mL), NEt3 (1.5 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated acetone and methanol mixed solution gave yellow crystals. Yield: 73.9 mg, 62%.1H NMR (CD3CN, 298 K): δ = 8.90 (s, 2H, NH), 8.04 (d, 4H, J = 9 Hz, aromatic ring), 7.92 (d, 4H, J = 9 Hz, aromatic ring), 7.48 (d, 4H, J = 9 Hz, aromatic ring), 7.18–6.85 (m, 64H, aromatic ring), 3.94 (s, 6H, CH3), 3.24 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −6.04 (s). 19F NMR (CD3CN, 298 K): δ = −151.65 (s, 10BF4−), −151.70 (s, 11BF4−). IR (KBr, cm−1): ν = 3371 (N–H), 2246 (C
CC6H4-4-NHC(O)C6H4-4-OCH3)2]BF4 (4·BF4). To a solution of [Cu2(μ-dppm)2(CH3CN)4](BF4)2 (114.8 mg, 0.093 mmol) and L4 (31.6 mg, 0.13 mmol) in degassed CH3CN (50 mL), NEt3 (1.5 mL) was added. The mixture was stirred overnight under nitrogen. After evaporation to dryness, the solid residue was collected and washed with water and diethyl ether. Subsequent diffusion of diethyl ether into the concentrated acetone and methanol mixed solution gave yellow crystals. Yield: 73.9 mg, 62%.1H NMR (CD3CN, 298 K): δ = 8.90 (s, 2H, NH), 8.04 (d, 4H, J = 9 Hz, aromatic ring), 7.92 (d, 4H, J = 9 Hz, aromatic ring), 7.48 (d, 4H, J = 9 Hz, aromatic ring), 7.18–6.85 (m, 64H, aromatic ring), 3.94 (s, 6H, CH3), 3.24 (s, 6H, CH2). 31P NMR (CD3CN, 298 K): δ = −6.04 (s). 19F NMR (CD3CN, 298 K): δ = −151.65 (s, 10BF4−), −151.70 (s, 11BF4−). IR (KBr, cm−1): ν = 3371 (N–H), 2246 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1662 (C
C), 1662 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS: m/z = 1844 [M]+. Anal. calcd for C107H90Cu3BF4N2O4P6 (%): C, 66.55; H, 4.70; N, 1.45. Found: C, 66.52; H, 4.71; N, 1.46.
O). ESI-MS: m/z = 1844 [M]+. Anal. calcd for C107H90Cu3BF4N2O4P6 (%): C, 66.55; H, 4.70; N, 1.45. Found: C, 66.52; H, 4.71; N, 1.46.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) CC6H4-4-NHC(O)C6H4-4-OCH3)2F]∞ (4·F). To a solution of 4·BF4 (76.8 mg, 0.062 mmol) in CH3CN, NBu4F (102.3 mg, 0.39 mmol) in CH3CN was added dropwise. The mixture was stirred overnight. The yellow precipitate was collected and washed by acetonitrile. Subsequent diffusion of diethyl ether into the concentrated CH3OH solution gave pale yellow crystals. Yield: 9.0 mg, 12%. 1H NMR (DMSO-d6, 298 K): δ = 8.12 (d, 4H, J = 9 Hz, aromatic ring), 7.92 (d, 4H, J = 9 Hz, aromatic ring), 7.26 (d, 4H, J = 9 Hz, aromatic ring), 7.20–6.83 (m, 64H, aromatic ring), 3.87 (s, 6H, CH3), 3.15 (s, 6H, CH2). 31P NMR (DMSO-d6, 298 K): δ = −6.04 (s). IR (KBr, cm−1): ν = 3429 (N–H), 2291 (C
CC6H4-4-NHC(O)C6H4-4-OCH3)2F]∞ (4·F). To a solution of 4·BF4 (76.8 mg, 0.062 mmol) in CH3CN, NBu4F (102.3 mg, 0.39 mmol) in CH3CN was added dropwise. The mixture was stirred overnight. The yellow precipitate was collected and washed by acetonitrile. Subsequent diffusion of diethyl ether into the concentrated CH3OH solution gave pale yellow crystals. Yield: 9.0 mg, 12%. 1H NMR (DMSO-d6, 298 K): δ = 8.12 (d, 4H, J = 9 Hz, aromatic ring), 7.92 (d, 4H, J = 9 Hz, aromatic ring), 7.26 (d, 4H, J = 9 Hz, aromatic ring), 7.20–6.83 (m, 64H, aromatic ring), 3.87 (s, 6H, CH3), 3.15 (s, 6H, CH2). 31P NMR (DMSO-d6, 298 K): δ = −6.04 (s). IR (KBr, cm−1): ν = 3429 (N–H), 2291 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1654 (C
C), 1654 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS: m/z = 1844 [M]+. Anal. calcd for C107H90Cu3FN2O4P6 (%): C, 68.97; H, 4.87; N, 1.50. Found: C, 68.90; H, 4.85; N, 1.51.
O). ESI-MS: m/z = 1844 [M]+. Anal. calcd for C107H90Cu3FN2O4P6 (%): C, 68.97; H, 4.87; N, 1.50. Found: C, 68.90; H, 4.85; N, 1.51.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (20971131 and J1103305), the Natural Science Foundation of Guangdong Province (S2012010010566), and the Undergraduate Innovative Experiment Program of Sun Yat-sen University (201504020055). We thank Dr Xiao-Long Feng for crystallographic data collection.Notes and references
- N. Busschaert, C. Caltagirone, W. V. Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed.
- F. Wang, L. Wang, X. Chen and J. Yoon, J. Chem. Soc. Rev., 2014, 43, 4312–4324 RSC.
- P. A. Gale, N. Busschaert, C. J. E. Haynes, L. E. Karagiannidis and I. L. Kirby, Chem. Soc. Rev., 2014, 43, 205–241 RSC.
- L. Fabbrizzi and A. Poggi, Chem. Soc. Rev., 2013, 42, 1681–1699 RSC.
- K. Bowman-James, A. Bianchi and E. García-Espana, Anion Coordination Chemistry, Wiley, New York, 2012 Search PubMed.
- P. A. Gale, Acc. Chem. Res., 2011, 44, 216–226 CrossRef CAS PubMed.
- V. Amendola, D. Esteban-Gómez, L. Fabbrizzi and M. Licchelli, Acc. Chem. Res., 2006, 39, 343–353 CrossRef CAS PubMed.
- K. Bowman-James, Acc. Chem. Res., 2005, 38, 671–678 CrossRef CAS PubMed.
- C. H. Park and H. E. Simmons, J. Am. Chem. Soc., 1968, 90, 2431–2432 CrossRef CAS.
- J. M. Lehn, Acc. Chem. Res., 1978, 11, 49–57 CrossRef CAS.
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS.
- M. R. Sambrook, P. D. Beer, J. A. Wisner, R. L. Paul, A. R. Cowley, F. Szemes and M. G. B. Drew, J. Am. Chem. Soc., 2005, 127, 2292–2302 CrossRef CAS PubMed.
- M. R. Sambrook, P. D. Beer, J. A. Wisner, R. L. Paul and A. R. Cowley, J. Am. Chem. Soc., 2004, 126, 15364–15365 CrossRef CAS PubMed.
- J. A. Wisner, P. D. Beer, N. G. Berry and B. Tomapatanaget, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4983–4986 CrossRef CAS PubMed.
- J. A. Wisner, P. D. Beer, M. G. B. Drew and M. R. Sambrook, J. Am. Chem. Soc., 2002, 124, 12469–12476 CrossRef CAS PubMed.
- G. W. Bates, P. A. Gale and M. E. Light, Chem. Commun., 2007, 21, 2121–2123 RSC.
- S. J. Brooks, P. A. Gale and M. E. Light, CrystEngComm, 2005, 7, 586–591 RSC.
- C. R. Bondy, P. A. Gale and S. J. Loeb, J. Am. Chem. Soc., 2004, 126, 5030–5031 CrossRef CAS PubMed.
- S. J. Coles, J. G. Frey, P. A. Gale, M. B. Hursthouse, M. E. Light, K. Navakhun and G. L. Thomas, Chem. Commun., 2003, 568–569 RSC.
- P. Byrne, G. O. Lloyd, K. M. Anderson, N. Clarke and J. W. Steed, Chem. Commun., 2008, 3720–3722 RSC.
- J. M. Russell, A. D. M. Parker, I. Radosavljevic-Evans, J. A. K. Howard and J. W. Steed, CrystEngComm, 2006, 8, 119–122 RSC.
- C. A. llioudis, D. A. Tocher and J. W. Steed, J. Am. Chem. Soc., 2004, 126, 12395–12402 CrossRef PubMed.
- K. J. Wallace, W. J. Belcher, D. R. Turner, K. F. Syed and J. W. Steed, J. Am. Chem. Soc., 2003, 125, 9699–9715 CrossRef CAS PubMed.
- R. Custelcean, P. V. Bonnesen, N. C. Duncan, X. H. Zhang, L. A. Watson, G. van Berkel, W. B. Parson and B. P. Hay, J. Am. Chem. Soc., 2012, 134, 8525–8529 CrossRef CAS PubMed.
- R. Custelcean, A. Bock and B. A. Moyer, J. Am. Chem. Soc., 2010, 132, 7177–7185 CrossRef CAS PubMed.
- R. Custelcean, J. Bosano, P. V. Bonnesen, V. Kertesz and B. P. Hay, Angew. Chem., Int. Ed., 2009, 48, 4025–4029 CrossRef CAS PubMed.
- R. Custelcean, P. Remy, P. V. Bonnesen, D. E. Jiang and B. A. Moyer, Angew. Chem., Int. Ed., 2008, 47, 1866–1870 CrossRef CAS PubMed.
- J. Wang, S. Li, P. Yang, X. Huang, X. J. Yang and B. Wu, CrystEngComm, 2013, 15, 4540–4548 RSC.
- S. G. Li, M. Y. Wei, X. J. Huang, X. J. Yang and B. Wu, Chem. Commun., 2012, 48, 3097–3099 RSC.
- S. G. Li, C. D. Jia, B. Wu, Q. Luo, X. J. Huang, Z. W. Yang, Q. S. Li and X. J. Yang, Angew. Chem., Int. Ed., 2011, 50, 5721–5724 CrossRef CAS PubMed.
- C. D. Jia, B. A. Wu, S. G. Li, X. J. Huang, Q. L. Zhao, Q. S. Li and X. J. Yang, Angew. Chem., Int. Ed., 2011, 50, 486–490 CrossRef CAS PubMed.
- Z. W. Yang, B. Wu, X. J. Huang, Y. Y. Liu, S. G. Li, Y. N. Xia, C. D. Jia and X. J. Yang, Chem. Commun., 2011, 47, 2880–2882 RSC.
- M. Wenzel, J. R. Hiscock and P. A. Gale, Chem. Soc. Rev., 2012, 41, 480–520 RSC.
- V. Amendola, L. Fabbrizzi and L. Mosca, Chem. Soc. Rev., 2010, 39, 3889–3915 RSC.
- R. Vilar, Eur. J. Inorg. Chem., 2008, 357–367 CrossRef CAS PubMed.
- P. A. Gale, Acc. Chem. Res., 2006, 39, 465–475 CrossRef CAS PubMed.
- H. Maeda and Y. Kusunose, Chem.–Eur. J., 2005, 11, 5661–5666 CrossRef CAS PubMed.
- W. L. Leong and J. J. Vittal, Chem. Rev., 2011, 111, 688–764 CrossRef CAS PubMed.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- H. Juwarker and K. S. Jeong, Chem. Soc. Rev., 2010, 39, 3664–3674 RSC.
- J. Sánchez-Quesada, C. Seel, P. Prados and J. de Mendoza, J. Am. Chem. Soc., 1996, 118, 277–278 CrossRef.
- J. W. Steed, Chem. Soc. Rev., 2009, 38, 506–519 RSC.
- S. K. L. Siu, C. C. Ko, V. K. M. Au and V. W. W. Yam, J. Cluster Sci., 2014, 25, 287–300 CrossRef CAS.
- V. W. W. Yam, K. K. W. Lo and K. M. C. Wong, J. Organomet. Chem., 1999, 578, 3–30 CrossRef CAS.
- V. W. W. Yam, K. K. W. Lo, W. K. M. Fung and C. R. Wang, Coord. Chem. Rev., 1998, 171, 17–41 CrossRef CAS.
- V. W. W. Yam, W. K. M. Fung and K. K. Cheung, Angew. Chem., Int. Ed., 1996, 35, 1100–1102 CrossRef CAS PubMed.
- V. W. W. Yam, W. K. Lee and T. F. Lai, Organometallics, 1993, 12, 2383–2387 CrossRef CAS.
- J. Diéz, M. P. Gamasa, J. Gimeno, A. Aguirre and S. García-Granda, Organometallics, 1991, 10, 380–382 CrossRef.
- M. Zhang, B. C. Su, C. L. Li, Y. Shen, C. K. Lam, X. L. Feng and H. Y. Chao, J. Organomet. Chem., 2011, 696, 2654–2659 CrossRef CAS PubMed.
- P. Dydio, D. Lichosyt and J. Jurczak, Chem. Soc. Rev., 2011, 40, 2971–2985 RSC.
- D. Voet, J. G. Voet and C. W. Pratt, Fundamentals of Biochemistry, Wiley, New York, 1999 Search PubMed.
- C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520–563 RSC.
- D. W. Yoon, H. Hwang and C. H. Lee, Angew. Chem., Int. Ed., 2002, 41, 1757–1759 CrossRef CAS.
- K. Chellappan, N. J. Singh, I. C. Hwang, J. W. Lee and K. S. Kim, Angew. Chem., Int. Ed., 2005, 44, 2899–2903 CrossRef CAS PubMed.
- K. Sato, S. Arai and T. Yamagishi, Tetrahedron Lett., 1999, 40, 5219–5222 CrossRef CAS.
- H. Ihm, S. Yun, H. G. Kim, J. K. Kim and K. S. Kim, Org. Lett., 2002, 4, 2897–2900 CrossRef CAS PubMed.
- R. Vargas, J. Garza, D. A. Dixon and B. P. Hay, J. Am. Chem. Soc., 2000, 122, 4750–4755 CrossRef CAS.
- V. S. Bryantsev and B. P. Hay, J. Am. Chem. Soc., 2005, 127, 8282–8283 CrossRef CAS PubMed.
- H. Y. Shi, J. Qi, Z. Z. Zhao, W. J. Feng, Y. H. Li, L. Sun, Z. J. Lin and H. Y. Chao, New J. Chem., 2014, 38, 6168–6175 RSC.
- J. C. Slater, J. Chem. Phys., 1964, 41, 3199–3204 CrossRef CAS PubMed.
- R. Nast, Coord. Chem. Rev., 1982, 47, 89–124 CrossRef CAS.
- D. Esteban-Gomez, L. Fabbrizzi and M. Liechelli, J. Org. Chem., 2005, 70, 5717–5720 CrossRef CAS PubMed.
- M. Boiocchi, L. DelBoca, D. Esteban-Gomez, L. Fabbrizzi, M. Licchelli and E. Monzani, Chem.–Eur. J., 2005, 11, 3097–3104 CrossRef CAS PubMed.
- M. Boiocchi, L. DelBoca, D. E. Gomez, L. Fabbrizzi, M. Licchelli and E. Monzani, J. Am. Chem. Soc., 2004, 126, 16507–16514 CrossRef CAS PubMed.
- X. He, F. Herranz, E. C. Cheng, R. Vilar and V. W. W. Yam, Chem.–Eur. J., 2010, 16, 9123–9131 CrossRef CAS PubMed.
- A. B. Descalzo, K. Rurack, H. Weisshoff, R. Martínez-Máñez, M. D. Marcos, P. Amorós, K. Hoffmann and J. Soto, J. Am. Chem. Soc., 2004, 127, 184–200 CrossRef PubMed.
- C. Jia, B. Wu, J. Liang, X. Huang and X. Yang, J. Fluoresc., 2010, 20, 291–297 CrossRef CAS PubMed.
- J. Diéz, M. P. Gamasa, J. Gimeno, A. Tiripicchio and M. T. Gamellini, J. Chem. Soc., Dalton Trans., 1987, 1275–1278 RSC.
- J. N. Demas and G. A. Crosby, J. Phys. Chem., 1971, 75, 991–1024 CrossRef.
- G. M. Sheldrick, SHELX 97, Program for Crystal Structure Solution and Refinement, Göttingen University, 1997 Search PubMed.
- V. A. Blatova, IUCr Comp. Comm. Newslett., 2006, 7, 4–38 Search PubMed.
- V. A. Blatova and D. M. Proserpio, Acta Crystallogr., Sect. A: Found. Crystallogr., 2009, 65, 202–212 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic files in CIF format for complexes 1·BF4–4·BF4, 1·PF6, 1·ClO4 and 4·F. Additional figures and tables. CCDC 1421592–1421598. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra18534c |
| This journal is © The Royal Society of Chemistry 2015 |