DOI:
10.1039/C5RA18502E
(Paper)
RSC Adv., 2016,
6, 745-757
Efficient removal of transition metal ions using poly(amidoxime) ligand from polymer grafted kenaf cellulose†
Received
10th September 2015
, Accepted 14th December 2015
First published on 17th December 2015
Abstract
A desired copolymer, cellulose-graft-polyacrylonitrile was synthesized by a free radical initiating process and optimum reaction conditions were determined for maximum grafting yield (125%). The nitrile functionalities of the grafted copolymers were converted into the amidoxime ligand by oximation reaction. The kenaf cellulose, cellulose grafted copolymer and poly(amidoxime) ligand were characterized by infrared spectroscopy, field emission scanning electron microscopy, transmission electron microscopy and X-ray photoelectron spectroscopy. The pH of the solution acts as a key factor to achieve the optical detection of metal ions using complexation of this ligand with some transition metal ions. The reflectance spectra of the [M–ligand]n+ complex was found to have a highest absorbance ranging from 97 to 99.9% at pH 6. The reflectance spectra were increased by increasing Cu2+ ion concentration and a broad peak at 700 nm was observed which indicates the charge transfer (π–π transition) complex. The adsorption capacity with copper was found to be superior; 326.6 mg g−1. The adsorption capacities for other transition metal ions were also found to be strong; Fe3+, Co3+, Mn2+, Cr3+, Ni2+ and Zn2+ were 273.6, 271.6, 241.7, 228.2, 204.2 and 224.3 mg g−1, respectively at pH 6. The experimental data of all metal ions fitted significantly with the pseudo-second-order rate equation. The data proved that the transition metal ion sorption onto ligands was well fitted with the Langmuir isotherm model (R2 > 0.99), which suggest that the cellulose-based adsorbent known as poly(amidoxime) ligand surface is homogenous and monolayer. The reusability was checked by the sorption/desorption process for seven cycles and the sorption and extraction efficiency in each cycle was determined. The new type of adsorbent can be reused in many cycles without any significant loss in its original sensing and removal performances.
Introduction
Cellulose is a biodegradable natural polymer that could be chemically modified to achieve efficient adsorption of toxic metal ions.1 The cellulose backbone could be tailored with chelating or metal binding functionalities through attachments and modification of the primary or secondary hydroxyl groups. The grafting of selected monomers to the cellulose backbone via direct attachment and the subsequent functionalization of the grafted copolymer chains with known chelating moieties is an interesting and cost-effective method. The grafted copolymer is recognized as the side chains covalently attached to the main chain of the cellulose backbone in ionic or free radical initiating process.2,3 The initiator created radicals at various sites on the cellulose backbone are highly reactive and ceric ion (Ce4+) has a profound effect on the initiator because of its high efficiency in grafting.3 However, biodegradation is a serious problem for natural polymers for long term applications in the adsorption processes.3 Cellulose possess good chemical stability and mechanical strength and these properties could be further improved by using grafting polymers onto cellulose and their subsequent transformation into known chelating ligands for the formation of metal ions complexes, an essential step in chelating mechanism.4
Global warming, climate change, ice melting, sea level rise and human activities have drastically reduced the availability of fresh water for human utilization. However, increased volume of pure water is an indisputable and to address this point various water treatment technologies have been proposed and applied at experiments and field levels. However, there is still a need for new technologies for wastewater treatment to meet the current regulatory limit of various metal ions discharge into the water-bodies. Contamination with toxic metal ions are found noticeable in the aqueous wastes from many industrial discharges from metal plating, mining, tanneries, chloro-alkali, radiator manufacturing, smelting, alloy industries and storage battery industries.5 Several measures have been taken to prevent the environmental pollution. For example, toxic metal ions have been removed by such as precipitation, adsorption, ion exchange and reverse osmosis have been applied to remove metal ion toxins.5 In some cases, wastewater treatment by precipitation has been followed by adsorption onto activated carbons to metal ions removal at highest level.6 Although, it seems to be quite effective in treating industrial effluents, chemical coagulation often induces secondary pollution by added chemical substances.6 Toxic metal precipitation also produces intractable sludge that must be treated often with high disposing cost.6,7 This drawback, together with the need for effective treatment is essential for the industrial effluents.
Here we extracted pure white cellulose from the kenaf fibre using conventional method and grafted it with acrylonitrile followed by chemical modification using free radical initiation method. The grafting of acrylonitrile onto kenaf cellulose under various experimental conditions and characteristic of grafting parameters were determined. Further, we synthesized a new poly(amidoxime) chelating ligand from the kenaf cellulose-graft-poly(acrylonitrile) (PAN) which apply for transition metal ions removal from water.
Experimental
Materials
Kenaf fibre was obtained from National Kenaf and Tobacco Board at Kuantan, Pahang, Malaysia and was cut into small pieces (∼0.5 cm in length) (see Fig. S1a of ESI†). The raw fibre (100 g) was boiled with 17% NaOH (800 mL) for 4 h and was washed with distilled water. The resultant product was boiled with glacial acetic acid (800 mL) for 1 h and washed with distil water. The dark colour kenaf cellulose was bleached with hydrogen peroxide (300 mL) and 7% NaOH (500 mL), washed with distilled water (500 mL) for several times and oven dried at 50 °C before use (see Fig. S1b of ESI†). Acrylonitrile monomer purchased from Aldrich and monomer was passed through columns filled with chromatographic grade activated alumina to remove inhibitors. Other chemicals such as ceric ammonium nitrate (CAN) (Sigma-Aldrich), methanol (Merck), sulphuric acid (Lab Scan), metal salts and other analytical grade reagents were used without purification.
Graft copolymerization
Exactly 6 g of kenaf cellulose was added into 600 mL distilled water. The reactions were carried out in 1 L three-neck round bottom flask fixed with stirrer and condenser in thermostat water bath. The N2 gas was purged into the flask to remove oxygen during the grafting process. The mixture was heated to 55 °C with stirring and 2.1 mL of diluted sulphuric acid (50%) was added to the mixture. After 5 min, 1.80 g of CAN (10 mL solution) was added and the reaction mixture was stirred under N2 gas. After 20 min, 16 mL acrylonitrile purified monomer was added into the cellulose suspension and stirred for 2 h under nitrogen. At the end of reaction, the mixture was cooled and was precipitated in excess amount of methanol and washed several times with methanolic solution (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water:4
water:4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The product was finally oven dried at 50 °C to a constant weight.7
1). The product was finally oven dried at 50 °C to a constant weight.7
Determination of grafting fractions
The grafting product was weighed and the homopolymer was extracted using Soxhlet purification with DMF for 12 h. The purified grafted copolymer was dried at 50 °C to a constant weight (see Fig. S1c of ESI†). The percentage of grafting (Gp) was determined using the following eqn (1):| |
 | (1) |
where W1 is the weight of parent polymer (cellulose) and W2 is the weight of grafted polymer (polyacrylonitrile).
The percentage of grafted fractions in the final graft copolymer was determined based on the amount of nitrogen content in the grafted samples as determined by Vario Marco Cube Elemental analyser. Grafting fraction of polyacrylonitrile (PAN) onto kenaf cellulose was calculated using the following eqn (2):7
| |
 | (2) |
where 53 is the molecular weight of repeating unit of PAN and 14 is the atomic mass of nitrogen.
Synthesis of poly(amidoxime) ligand
Hydroxylamine solution was prepared by 20 g of hydroxylamine hydrochloride (NH2OH·HCl) dissolved in 500 mL of methanolic solution (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water; 4
water; 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). About 50% of NaOH solution was added in cold condition and the precipitate form of NaCl was removed using filtration. The pH of the reaction was adjusted to pH 11 by NaOH solution. The ratio of methanol to water was maintained at 4
1). About 50% of NaOH solution was added in cold condition and the precipitate form of NaCl was removed using filtration. The pH of the reaction was adjusted to pH 11 by NaOH solution. The ratio of methanol to water was maintained at 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v). Then poly(acrylonitrile) grafted kenaf cellulose (10 g) was placed into a two-neck round bottom flask fixed with a stirrer, condenser and thermostat water bath.7,8 The prepared hydroxylamine solution was then added to the flask and the reaction was carried out at 70 °C for 6 h. After completion of reaction, the chelating polymeric ligand was separated from hydroxylamine solution by filtration followed by washing with methanolic solution (methanol
1 (v/v). Then poly(acrylonitrile) grafted kenaf cellulose (10 g) was placed into a two-neck round bottom flask fixed with a stirrer, condenser and thermostat water bath.7,8 The prepared hydroxylamine solution was then added to the flask and the reaction was carried out at 70 °C for 6 h. After completion of reaction, the chelating polymeric ligand was separated from hydroxylamine solution by filtration followed by washing with methanolic solution (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water/4
water/4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The ligand was treated with 100 mL of 0.1 M HCl in methanolic solution 5 min for conversion into H-form ligand. The ligand was filtered and washed several times with methanol and dried at 50 °C to a constant weight (see Fig. S1d of ESI†).
1). The ligand was treated with 100 mL of 0.1 M HCl in methanolic solution 5 min for conversion into H-form ligand. The ligand was filtered and washed several times with methanol and dried at 50 °C to a constant weight (see Fig. S1d of ESI†).
Optical sensing of metal ions
For a typical optical metal ions sensing, 150 mg of the polymeric ligand was immersed into 10 mL of buffer solution which is adjusted to appropriate pH from 2 to 9 (0.1 M sodium acetate with acetic acid). Then metal ions (Cu2+, Fe3+, Co3+, Cr3+ and Ni2+) solution concentration of 6 mg L−1 was added to each pH solution with shaking in a temperature controlled shaker machine (Lab companion, SI-600) at 30 °C for 2 h at a constant agitation speed of 180 rpm to achieve good colour separation. In case of colour optimization, 150 mg of the polymeric ligand was also immersed into 10 mL of acetate buffer at pH 6 and metal ions solution (10 mL of each metal ions) concentration of 6, 12 and 18 mg L−1 was added at constant volume (20 mL) with shaking in a similar manner for 2 h at a constant agitation speed of 180 rpm to achieve good colour separation. A bank solution was also prepared following the same procedure for comparison of colour formation and detection.9,10 After equilibration, the solid ligand was separated using filtration and dried the ligand at 50 °C for 2 h. The optical colour assessment and absorbance were measured by solid state UV-vis NIR spectrophotometer (UV-2600 Shimadzu).
Batch adsorption
In case of removal experiments for a typical transition metal ions (Cu2+, Fe3+, Co3+, Mn2+, Cr3+, Ni2+ and Zn2+), exactly 150 mg of the polymeric ligand was also immersed into metal ion (single metal) solution of 10 mL (0.1 M) to appropriate pH (3 to 6) using sodium acetate buffer (10 mL) and shaking for 2 h with the speed of 180 rpm. After equilibration, the ligand was separated by filtration and metal ions concentration were determined by ICP-OES (Perkin Elmer, Optima 8300). The initial and final readings (after adsorption) of the metal ion concentration were calculated according to eqn (3).| |
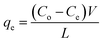 | (3) |
here, qe is the equilibrium adsorption amount (mg g−1), Co is the initial concentration of metal solution (mg L−1), Ce is the equilibrium concentration of metal (mg L−1) after adsorption, V is the volume of metal solution (L), L is the mass of polymeric ligand (g).
In case of trace level of metal ions removal experiment, 5 mL of 10 ppm of metal ions (single metal) solution at pH 6 (sodium acetate buffer) was used with 150 mg of dried ligand, shaking for 2 h with the speed of 180 rpm. The initial metals solution and final solution (after adsorption) was analysed by ICP-MS (Agilent 7500 series) and analysis was carried out according to eqn (3).
In case of isotherm experiments, batch adsorption experiments (Cu2+, Fe3+, Co3+, Cr3+ and Ni2+) were performed using the traditional bottle-point method with constant temperature at 30 °C and 180 rpm agitation by shaker machine (Lab companion, SI-600). Exactly 150 mg of the adsorbent samples of the poly(amidoxime) ligand were equilibrated with 20 mL of aqueous solutions containing metal ions (single metal) for 2 h. In the isothermal study, the initial concentration of metal ions ranged from 5 ppm to 1300 ppm. The initial metals solution and final solution (after adsorption) was analysed by ICP-MS (Agilent 7500 series) and analysis was carried out according to eqn (3).
Kinetic study
Sorption kinetic study was carried out with 150 mg of ligand immersed into 10 mL of 0.1 M metal ion (single metal) and 10 mL of acetate buffer at pH 6, shaking with the speed of 180 rpm at various time intervals such as 2, 5, 10, 20, 30, 60 and 120 min, and the metal ions concentrations were estimated by ICP-OES (Perkin Elmer, Optima 8300). The residual metal concentration was determined using the final concentration (after adsorption) is deducted from initial metal ions concentration, as calculated by eqn (4):| |
 | (4) |
here, qt is the adsorption amount at time t (mg g−1), Co is the initial concentration of metal solution (mg L−1) and Ct (mg L−1) is metal concentration at time t.
Results
FT-IR analysis
The synthesized chelating ligand, kenaf cellulose and cellulose grafted copolymer were characterized by infrared (IR) spectra using FT-IR spectrometer (Perkin-Elmer). IR spectrum of kenaf cellulose showed adsorption bands at 3438 and 2921 cm−1 which were referred to O–H and C–H stretching, respectively (Fig. 1a). The band at 1638 cm−1 was due to the bending mode of the absorbed water.11 A smaller band at 1426 cm−1 was observed for the CH2 symmetric bending. The absorbance at 1376 and 1160 cm−1 originated from the O–H bending and C–O stretching, respectively. The C–O–C pyranose ring skeletal vibration produced a strong band at 1065 cm−1. A small sharp peak at 895 cm−1 corresponded to the glycosidic C1–H deformation with ring vibration contribution and OH bending, which is characteristic of α-glycosidic linkages between glucose units in cellulose.11 The IR spectrum of purified polyacrylonitrile grafted cellulose (kenaf-g-PAN) showed new absorption band at 2244 cm−1 due to CN stretching of acrylonitrile (Fig. 1b) and other bands retained from kenaf cellulose (3438, 2921, 1638, 1426, 1376, 1160, 1065 and 895 cm−1). The amidoxime functional group from poly(amidoxime) ligand showed new absorption bands at 1680 and 1648 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching and N–H bending modes, respectively (Fig. 1c). In addition, a shoulder created at 3304 cm−1 for both N–H and OH stretching bands and 1401 cm−1 for OH bending was observed (Fig. 1c). It was found that the CN band for 2244 cm−1 disappeared and the new absorption bands for amidoxime group appeared at 3304 cm−1, 1680 cm−1 stretching and 1401 cm−1 bending modes, which the confirmed the of successful synthesis of amidoxime ligand from polyacrylonitrile grafted kenaf cellulose.
N stretching and N–H bending modes, respectively (Fig. 1c). In addition, a shoulder created at 3304 cm−1 for both N–H and OH stretching bands and 1401 cm−1 for OH bending was observed (Fig. 1c). It was found that the CN band for 2244 cm−1 disappeared and the new absorption bands for amidoxime group appeared at 3304 cm−1, 1680 cm−1 stretching and 1401 cm−1 bending modes, which the confirmed the of successful synthesis of amidoxime ligand from polyacrylonitrile grafted kenaf cellulose.
 |
| | Fig. 1 FTIR spectra of (a) kenaf cellulose, (b) poly(acrylonitrile) grafted kenaf cellulose and (c) poly(amidoxime) chelating ligand. | |
The evidence of metal–ligand complexes was determined by IR spectra as shown in Fig. 2 for poly(amidoxime) ligand and other metal–ligand complexes. It was clearly seen that both N–H and OH stretching mode of ligand (3304 cm−1) were affected by metal–ligand complexes. In addition, 1680 and 1648 cm−1 of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching and N–H bending modes were larger due to the metal–ligand complex ring formation, which is a strong evidence for adsorption occurred by metal ions on the cellulose-based polymeric chelating ligand.
N stretching and N–H bending modes were larger due to the metal–ligand complex ring formation, which is a strong evidence for adsorption occurred by metal ions on the cellulose-based polymeric chelating ligand.
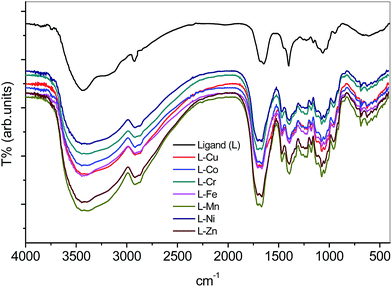 |
| | Fig. 2 FTIR spectra of poly(amidoxime) ligand (top black line) and metal–ligand complexes (various coloured lines). | |
FE-SEM, HR-TEM and BET analysis
FE-SEM measurement was performed with JEOL (JSM-7800F). The SEM micrograph of the kenaf cellulose showed wooden stick like smooth morphologies (Fig. 3a). The SEM micrograph of the poly(acrylonitrile) grafted kenaf cellulose showed that distinguishable grafting occurred on the surface of wooden stick like cellulosic structure having rough surface surrounding the stick due to PAN grafting (Fig. 3b). An enlarged morphology of PAN grafting product is shown in Fig. 3c. The poly(amidoxime) ligand showed distinct morphologies with smaller size of spherical shape was found (Fig. 3d). The poly(amidoxime) ligand after adsorption with copper(II) metal ion showed bigger size of spherical shape (Fig. 3e), which is different in sizes. HR-TEM was measured with Hitachi instrument (HT-7700) and nanoscale micrograph (Fig. S2 at ESI†) showed scattered Cu(II)-complex (average 5 nm size) which is a strong evidence to the adsorption occurred by the polymeric ligand.
 |
| | Fig. 3 FE-SEM micrograph of (a) kenaf cellulose, (b) PAN-g-cellulose, (c) enlarged view of PAN-g-cellulose, (d) poly(amidoxime) ligand, (e) poly(amidoxime) ligand after adsorption of Cu2+, (f) nitrogen adsorption–desorption isotherms of poly(amidoxime) ligand. | |
Textural properties of the synthesized poly(amidoxime) ligand can be analysed using the isothermal nitrogen adsorption–desorption method. The pure poly(amidoxime) ligand exhibits a typical isotherm of type III with H3 hysteresis loop can be seen in Fig. 3f. A gradual increase in adsorption at relative pressures of 0.5–0.9 and a steep increase in adsorption at relative pressures of 0.9–0.99 attributed to the capillary nitrogen condensation in the polymer ligand (Fig. 3f). The BET surface areas, pore volumes, and average pore diameters are 118.7 m2 g−1, 0.53 cm3 g−1 and 11.3 nm, respectively. Although the surface area of this polymer ligand is smaller as compare to reported materials, it is still suitable to act as a promising sorbent considering the high density of functional groups on the accessible pore channels.
Reaction mechanism
A number of reports are available on the mechanism of grafting reaction of acrylic monomers with starch or cellulose materials using free radical initiation method.12,13 Recent studies have proposed an alternative mechanism of cellulose units containing primary OH groups in which metal ions form a free radical on the oxygen atom which reacts with vinyl or acrylic monomers for copolymerization reactions.14 In this study, kenaf cellulose is grafted with acrylonitrile using free radical chain reaction with the ceric ion as initiator (Scheme 1). The ceric(IV) ion forms a complex with the OH groups of glucose units in the kenaf cellulose and the hydrogen atom is oxidized by the reduction of Ce4+ ion to Ce3+ ion. Then the cellulose free radicals induced the initiation of grafting by the addition of double bond in acrylonitrile monomer. This results in the radical formation for propagation reaction. The termination reaction of the growing polymer chain of the cellulose-monomer molecules results in the combination of grafting is shown in Scheme 1, although termination by disproportionation is also possible.15
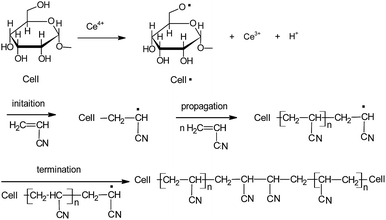 |
| | Scheme 1 Graft copolymerization of acrylonitrile onto kenaf cellulose (cell • is a glucose unit of cellulose). | |
Poly(amidoxime) ligand
The kenaf cellulose-g-polyacrylonitrile copolymer was synthesized from the reaction between kenaf cellulose with acrylonitrile monomer using free radical polymerization process. The optimum reaction conditions of graft copolymerization were found to be for cellulose (AGU), mineral acid (H2SO4), ceric ammonium nitrate (CAN) and acrylonitrile (AN) at 0.037, 0.034, 0.0054 and 0.503 mol L−1, respectively. Subsequently, the grafted copolymer having nitrile group was reacted with hydroxylamine for conversion of polymeric chelating ligand known as the poly(amidoxime) functional groups using Beckmann or Lossen type rearrangement (Scheme 2).
 |
| | Scheme 2 Kenaf cellulose-g-PAN converted into poly(amidoxime) ligand. | |
The conversion of ligand was reached to maximum 98.08% due to N content of 14.46% in the grafting product whereas N content was 17.89% in the polymeric ligand (theoretical value of N was 18.24% in the ligand). Hence, amidoxime functional group would participate to enhance the significant binding properties with metal ions. According to our previous works,15 optimum amidoximation was achieved with methanol to water ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using reaction pH 11 at 70 °C for 4 h. The H-form ligand was obtained after treatment with 0.1 M HCl solution and physical and chemical properties of poly(amidoxime) ligand were summarized in Table 1.
1 using reaction pH 11 at 70 °C for 4 h. The H-form ligand was obtained after treatment with 0.1 M HCl solution and physical and chemical properties of poly(amidoxime) ligand were summarized in Table 1.
Table 1 Physical and chemical properties of poly(amidoxime) ligand
| Parameter |
|
| Percentage of grafting (Gp) |
125 |
| Grafting fraction (Gf) |
132 |
| Swelling capacity (%) |
15 |
| Average exchange rate (t1/2 min) |
7 |
| Highest capacity (mg g−1) |
326.6 |
Optical detection of metal ions
Effect of solution pH. The effect of pH of the solution is a key factor for the selective and optical detection of metal ions.9,10 The pH of the solution is significant when the ligand was sensing the transition metal ions. The reflectance spectra of the [Cu–ligand]n+ complex was observed over a wide pH ranges from 2 to 9. The amount of Cu2+ ion adsorbed by polymeric ligand was adequate to attain good colour separation (signal) between the ligand (blank) and Cu2+ ion-sensing sample as shown in Fig. 4. The ligand was robust at pH 6–7 for its optical colour intensity and signal response for Cu2+ ions in which the highest absorbance was 99.9% at pH 6. The results suggest that the ligand has high functionality and affinity to Cu2+ ion at pH 6 using sodium acetate buffer solution (0.1 M sodium acetate solution adjusted pH by adding acetic acid). For other transition metal ions, [M–ligand]n+ showed similar behaviour as the highest absorbance were ranging from 97 to 99% at pH 6 (see Fig. S3 of ESI†). Therefore, pH 6 was chosen as optimum experimental condition in the optical recognition system for metals ions to get a high sensitive response. This finding justifies that the selective recognition of target metal ions by ligand supported adsorbent at a specific pH region is the important factor for selective metals ions capturing.9,10
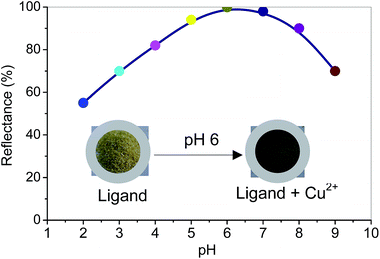 |
| | Fig. 4 Effect of solution pH for Cu2+ ions sensing by polymeric ligand (150 mg) at various pH with 6 mgL−1 of Cu2+ in 20 mL volume for 2 h. | |
Colour optimization
The polymeric ligand (adsorbent) exhibited high physical and textural properties for visual inspection of transition metal ions. The reflectance spectra was increased when increasing of Cu2+ ion concentration from 0 to 18 ppm at other experimental conditions were fixed as shown in Fig. 5. In addition, a broad peak at about 700 nm was created when Cu2+ ion is adsorbed by ligand whereas blank polymeric ligand does not show any peak at 700 nm. The reflectance spectra of the polymeric ligand exhibited a new peak at about 700 nm with addition of Cu2+ ion indicating the charge transfer (π–π transition) complex. In addition, other transition metal ions also developed new peak at 550, 640, 580 and 570 nm of Fe3+, Co3+, Cr3+ and Ni2+, respectively (see Fig. S4 of ESI†) and colour optimization of some metal ions is shown in Table 2. It is evident that the increasing absorbance corresponds to equilibrium colour formation between ligand and metal ions are sensitive determination in ultra-trace concentrations.9,10
 |
| | Fig. 5 Colour optimization with increasing concentrations of Cu(II) ions at pH 6 with reflectance spectra. | |
Table 2 Colour optimization with increasing concentrations of cobalt, iron, chromium and nickel ions at pH 6
| Metal ion |
0 ppm |
6 ppm |
10 ppm |
15 ppm |
| Co3+ |
 |
 |
 |
 |
| Cr3+ |
 |
 |
 |
 |
| Fe3+ |
 |
 |
 |
 |
| Ni2+ |
 |
 |
 |
 |
Adsorption of metal ions by ligand
Effect of pH for removal of metal ions. The pH effect on the metal ions adsorption behaviour by the poly(amidoxime) chelating ligand was evaluated over a pH ranges from 3 to 6 and pH was adjusted by adding sodium acetate buffer solution. Thus, adsorption behaviour of the polymeric ligand was determined by the binding of some transition metal ions. The analytical results render the metal ions adsorption capacities of the selected metal ions were found to be increase from pH 3 to 6. The ligand has high affinity to Cu2+ ion in neutral pH region, even other common transition metals showed higher affinity such as iron, cobalt, manganese and chromium at pH 6. The binding capacities of Cu2+, Fe3+, Co3+, Mn2+, Cr3+, Ni2+ and Zn2+ were 326.6, 273.6, 271.6, 241.7, 228.2, 204.2 and 224.3 mg g−1, respectively at pH 6 (Fig. 6). It was found that metal ions uptake by the ligand was pH-dependent. The adsorption capacity of the synthesized chelating ligand towards the metal ions was in the order of Cu2+ > Fe3+ > Co3+ > Mn2+ > Cr3+ > Zn2+ > Ni2+. The function of ligand molecule of polymeric adsorbent is actively working to make complexation with metal ions for removal from water. However, kenaf cellulose modified ligand is pH sensitive for target ions detection and removal.7–9,15
 |
| | Fig. 6 Metal ions adsorption capacity by the ligand as a function of pH. Reaction condition was 150 mg of dried ligand, 10 mL of 0.1 M sodium acetate buffer solution at pH 3–6, and 10 mL of 0.1 M metal ion solution shaken for 2 hours. | |
Highly coloured chelating ligand was observed after batch equilibration with the metal ions due to formation of complexes of amidoxime groups with the metal ions. The colour detection was obviously observed from the resin, adsorbed with some transition metal ions. The amidoximate anions are bidentate ligand trapped the metal ions and forming five-membered ring complexes. The metal ions are bound to both oxygen atoms and the amine group, the chelate complex is shown in Fig. 7.
 |
| | Fig. 7 Polymeric ligand with metal ions. | |
For comparison purpose, adsorption studies were carried out with metal ions at pH 6. The binding capacity (mg g−1) of kenaf cellulose, cellulose-graft-copolymer and poly(amidoxime) ligand are presented in Table S1 (see ESI†). It was found that metal ions uptake by the ligand was excellent whereas kenaf cellulose and cellulose-graft-copolymer showed very negligible binding affinity to all metal ions (Table S1†). It was also observed that binding capacity with cellulose grafting copolymer is less than kenaf cellulose due to nitrile groups with the grafting materials having hydrophobic characteristic which gives more hindrance to adsorption affinity.
Adsorption kinetic
The sorption by complexation reaction mechanisms are slowing kinetic to take up target ions than the ion-exchange and hydrogen bonding reaction mechanisms.9,10 Therefore, required contact time between metal ions (Cu2+, Fe3+, Co3+, Mn2+, Cr3+, Ni2+ and Zn2+) and polymeric ligand for selected metal ions removal was determined using a series of batch contact time experiments. The time depending on maximum metal ions sorption by the ligand was determined (filtrate solution analyzed by ICP-OES). Several metal ions were used to study the rate of adsorption in buffer solution at pH 6. Adsorption and efficiency of the adsorption is explained by the kinetic models for appropriate understanding of the mechanism of adsorption. The pseudo-first-order kinetic equation is widely used for the adsorption of solute from solution. The equation is given by:| |
 | (5) |
where qt and qe are the adsorption capacity at time t and at equilibrium (mg g−1), and k1 is the rate constant of the pseudo-first-order adsorption process (min−1). The values of qe and k1 can be determined from the intercept and slope of plots of log(qe − qt) versus t (Fig. 8) and corresponding values are shown in Table 3. Although R2 values are acceptable for all adsorbates however the experimental values of adsorption capacity (qm exp.) shows a significant difference compared to the calculated values (qe cal.) from the first-order plot (Table 3). These results suggest least fit of the pseudo-first-order model to the experimental data.
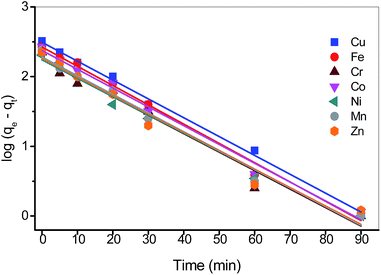 |
| | Fig. 8 Kinetic plots of pseudo-first-order model for transition metals on the poly(amidoxime) ligand. Other conditions: 0.1500 g of dried ligand, 10 mL of 0.1 M sodium acetate buffer, 10 mL of 0.1 M metal ion solution. | |
Table 3 Adsorption kinetic parameters of the pseudo-first-order model for transition metals on the poly(amidoxime) ligand
| Adsorbate |
Pseudo-first-order |
Experimental |
| qe (mg g−1) |
k1 (min−1) |
R2 |
qm (mg g−1) |
| Cu |
310.4 |
0.0270 |
0.996 |
326.6 |
| Fe |
266.3 |
0.0276 |
0.989 |
273.4 |
| Co |
237.5 |
0.0269 |
0.987 |
271.6 |
| Cr |
174.8 |
0.0265 |
0.970 |
228.2 |
| Ni |
174.6 |
0.0262 |
0.984 |
204.2 |
| Mn |
190.4 |
0.0267 |
0.983 |
241.7 |
| Zn |
180.6 |
0.0263 |
0.966 |
224.3 |
The pseudo-second-order model represents the adsorption rate relationship with the difference of adsorption capacities at equilibrium and at different contact times, the pseudo-second-order kinetic model can be expressed as:
| |
 | (6) |
where
k2 is the rate constant of the pseudo-second-order sorption (g mg
−1 min
−1) and
qe is the amount of metals adsorbed (mg g
−1) at equilibrium and
qt is the amount of the adsorption (mg g
−1) at any time
t. The values of
k2 and
qe can be calculated from a plot of
t/
qt versus t (
Fig. 9) and corresponding values are presented in
Table 4. A significant difference was found between the parameters depicted in
Tables 3 and
4. However,
Table 4 shows that the correlation coefficients for the pseudo-second-order adsorption are high and the calculated
qe values agree well with the experimental values. These results suggest that the second-order mechanism is predominant and that the chemical process is the adsorption mechanism due to the valance forces through sharing or exchanging electrons between the transition metal ions and the poly(amidoxime) ligand.
16,17
 |
| | Fig. 9 Kinetic plot of the pseudo-second-order model for transition metals on the poly(amidoxime) ligand. Other conditions: 0.1500 g of dried ligand, 10 mL of 0.1 M sodium acetate buffer, 10 mL of 0.1 M metal ion solution. | |
Table 4 Adsorption kinetic parameters of the pseudo-second-order model for transition metals on the poly(amidoxime) ligand
| Adsorbate |
Pseudo-second-order |
Experimental |
| qe (mg g−1) |
k2 (g mg−1 min−1) × 10−3 |
R2 |
qm (mg g−1) |
| Cu |
344.7 |
0.0242 |
0.975 |
326.6 |
| Fe |
284.8 |
0.0339 |
0.967 |
273.4 |
| Co |
282.3 |
0.0247 |
0.984 |
271.6 |
| Cr |
239.2 |
0.0217 |
0.990 |
228.2 |
| Ni |
209.6 |
0.0127 |
0.996 |
204.2 |
| Mn |
255.1 |
0.0218 |
0.990 |
241.7 |
| Zn |
243.9 |
0.0312 |
0.980 |
224.3 |
Sorption isotherms
The effect of metal ions concentration on the adsorption capacity and the sorption isotherm studies were carried out to find out comprehensive metal uptake by adsorbent. Transition metal ions (Cu2+, Fe3+, Co3+, Cr3+, Ni2+) was adsorbed by the cellulose-based polymeric ligand in which the initial concentration was increased from 5 to 1300 mL L−1 while ligand dose, pH and agitation period was constant. The results indicated that adsorption capacity increased with the increase in initial concentration of metal ions and adsorption value also gradually increased up to certain limit.
Nonlinear forms of the isotherm models
The Langmuir isotherm theory represented a saturated monolayer of solute molecules on the adsorbent surface with no migration of adsorbates molecules and a constant energy of adsorption.17 The nonlinear Langmuir isotherm model is expressed as eqn (7):| |
 | (7) |
where qe is the equilibrium adsorption capacity of metal ions on the adsorbent (mg g−1), qm is the maximum capacity of the adsorbent (mg g−1), KL, the Langmuir adsorption constant (L mg−1) and Ce represent the equilibrium concentration of metal ions in solution (mg L−1).
The Freundlich model describes the adsorption of a reversible heterogeneous surface, which can be applied to multilayer adsorption.16 The nonlinear empirical equation of Freundlich isotherm model is described as eqn (8):
where equilibrium values of
qe and
Ce are defined as
eqn (7),
KF is the Freundlich constant (L mg
−1) which indicates the adsorption capacity, and 1/
n is related to the heterogeneity factor and indicates the adsorption capacity. Here
n, gives the degree of non-linearity in which if
n = 1 the adsorption is linear, if
n < 1 the adsorption is nonlinear.
18 All the model parameters were evaluated by both nonlinear regression and linear least-squares method using Origin 8.0 software.
Fig. 10 shows Langmuir and Freundlich adsorption isotherms of the polymer adsorbents by nonlinear analysis and the values of corresponding isotherm parameters are shown in
Table 5. The values of maximum adsorption capacity determined using Langmuir model were 321.7, 278.6, 270.1, 224.8 and 201.1 mg g
−1 of Cu, Fe, Co, Cr and Ni, respectively. All these adsorption values obtained by Langmuir model are close to the experimental adsorbed amounts, which indicates that the modeling of Langmuir for the adsorption system is in good agreement.
 |
| | Fig. 10 Nonlinear fitting of Langmuir isotherms (solid lines) and Freundlich curves (dot lines); experimental conditions: initial metal ions concentration range 5–1300 mg L−1, sample dose 150 mg/20 mL, solution pH 6, temperature 32 °C, contact time 2 h. | |
Table 5 Langmuir and Freundlich isotherm parameters obtained by nonlinear fitting for the poly(amidoxime) ligand
| Adsorbate |
Langmuir |
Freundlich |
| qm (mg g−1) |
KL (L g−1) |
R2 |
n |
KF (L mg−1) |
R2 |
| Cu |
321.7 |
0.187 |
0.910 |
0.211 |
62.95 |
0.981 |
| Fe |
278.6 |
0.174 |
0.981 |
0.196 |
67.79 |
0.977 |
| Co |
270.1 |
0.165 |
0.955 |
0.186 |
51.57 |
0.965 |
| Cr |
224.8 |
0.140 |
0.892 |
0.160 |
49.99 |
0.987 |
| Ni |
201.1 |
0.128 |
0.989 |
0.147 |
51.57 |
0.975 |
Linear forms of the isotherm models
The Langmuir and the Freundlich isotherms are the most common isotherms models to describe and investigate the equilibrium data of adsorption from aqueous solution.16 The Langmuir isotherm model is derived to model the assumptions of monolayer adsorption, a certain number of identical active sites, active sites distributed evenly on the surface of the adsorbent and no interaction between adsorbents.16 The linear form of the Langmuir isotherm equations is known by the following eqn (9):| |
 | (9) |
where equilibrium values of qe and Ce are defined as eqn (7), while qm and KL represent the maximum adsorption capacity of adsorbents (mg g−1) and the Langmuir adsorption constant (L mg−1). The data were utilized according to Langmuir sorption isotherms model (linear from) by the plot of Ce/qe against Ce as shown in Fig. 11. The values of qm and KL are calculated from the slope and intercept of the linear plot of Ce/qe against Ce. The data suggested that the metal ions sorption onto ligand was well fitted with the Langmuir isotherm model as indicated by the R2 values (>0.99). The calculated data for the maximum sorption capacity (qm) and the sorption coefficient KL are presented in Table 6. The value of qm obtained from the linear plot of Langmuir isotherm was correlated with the measured qm and copper adsorption is higher than nickel including other transition metal ions which suggest that a monolayer adsorption has occurred by the polymeric ligand.
 |
| | Fig. 11 Linear fitting of Langmuir adsorption isotherms of metal ions; experimental conditions: initial metal ions concentration range 5–1300 mg L−1, sample dose 150 mg/20 mL, solution pH 6, temperature 32 °C, contact time 2 h. | |
Table 6 Langmuir and Freundlich isotherm parameters obtained by linear fitting for the poly(amidoxime) ligand
| Adsorbate |
Langmuir |
Freundlich |
| qm (mg g−1) |
KL (L g−1) |
R2 |
n |
KF (L mg−1) |
R2 |
| Cu |
308.6 |
0.014 |
0.990 |
2.755 |
6.910 |
0.960 |
| Fe |
282.4 |
0.015 |
0.993 |
2.661 |
8.204 |
0.974 |
| Co |
263.3 |
0.018 |
0.996 |
2.611 |
9.639 |
0.973 |
| Cr |
232.5 |
0.018 |
0.996 |
2.708 |
11.96 |
0.957 |
| Ni |
211.4 |
0.014 |
0.992 |
2.663 |
9.429 |
0.955 |
Linear forms of the isotherms models are also widely adopted to determine the isotherm parameters or the most fitted model for the adsorption system due to the mathematical simplicity. The Freundlich isotherm is best known as multilayer adsorption and adsorption on heterogeneous surfaces,16,17 by the following eqn (10):
| |
 | (10) |
where
KF and
n are the Freundlich constants, which is the adsorption capacity and the adsorption strength, respectively.
KF and
n can also be calculated from the intercept and the slope of the linear plot of log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus
qe versus log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce. The data were also utilized into Freundlich isotherms model by the plot of log of
qe versus Ce as shown in
Fig. 12. The
KF and
n are calculated and presented in
Table 6. The Freundlich equation shows that a non-significant correlation (
R2 < 0.95) with the experimental data, which suggest that no multilayer adsorption was occurred by selected transition metals. The experimental results of the Freundlich adsorption model with the experimental data were also presented in
Table 6.
 |
| | Fig. 12 Linear fitting of Freundlich isotherms for the adsorption of metal ions; experimental conditions: initial metal ions concentration range 5–1300 mg L−1, sample dose 150 mg/20 mL, solution pH 6, temperature 32 °C, contact time 2 h. | |
Comparison of maximum adsorption capacities (qm)
The comparison of maximum adsorption capacities (qm) of the adsorbents, which were calculated by both the linear and nonlinear Langmuir model according to reported method.18 It clearly shows that the values of qm obtained from nonlinear Langmuir model are very close to experimental values. On the other hand, the values of qm by the linear Langmuir model are increased for Fe, Cr and Ni whereas decreases for Cu and Co ions. Here, the differences (Da) between the qm derived from nonlinear Langmuir model and the experimental data are presented in Table 7. Similarly the differences (Db) between the qm derived from linear Langmuir model and the experimental data are also presented in Table 7. This indicates for this adsorption system of transition metal ions are coordinated with amidoxime-functionalized polymeric ligand for metals removal, the results derived from linear fitting of the isotherm models can cause discrepancy (Table 7).
Table 7 Comparison between the maximum adsorption capacities by the Langmuir nonlinear and linear isotherm modelsa
| Adsorbate |
Exp. measured |
Nonlinear Langmuir |
Linear Langmuir |
Da (mg g−1) |
Db (mg g−1) |
| qm (mg g−1) |
qm (mg g−1) |
qm (mg g−1) |
| Da means the differences between the maximum adsorption capacities of nonlinear model and experimental data, Db means the differences between the maximum adsorption capacities of linear model and experimental data. |
| Cu |
326.3 |
321.7 |
308.6 |
4.6 |
17.7 |
| Fe |
273.4 |
271.6 |
282.4 |
1.8 |
−9.0 |
| Co |
271.6 |
270.1 |
263.3 |
1.5 |
8.3 |
| Cr |
228.2 |
224.8 |
232.5 |
3.4 |
−4.3 |
| Ni |
204.2 |
201.1 |
211.4 |
3.1 |
−7.2 |
Adsorption mechanism of metal ions sorption by polymer ligand
The efficient adsorption of copper cations by amidoximated polymeric ligand is achieved by virtue of the strong chelating ability of the amidoxime groups, which act as bidentate ligands. The X-ray photoelectron spectra (Scanning X-ray Microprobe PHI Quantera II) are obtained to interpret the sorption mechanisms of Cu2+ on the polymeric ligand. The wide scan XPS spectra of polymer ligand after adsorption of copper which compared with those before sorption as shown in Fig. 13. The peaks at binding energies (BEs) of 284.0 eV, 399.3 eV, and 531.4 eV correspond to the C 1s, N 1s, and O 1s spectra, respectively, which exist in both adsorbed and unadsorbed samples (Fig. 13a and b). The adsorption of Cu2+ is evident by the appearance of two new peaks with BEs of 932.9 eV and 952.0 eV for the signals of Cu2p3/2 and Cu2p1/2 as shown in Fig. 13b.
 |
| | Fig. 13 Wide scan the X-ray photoelectron spectra of poly(amidoxime) ligand with copper sorption (a) and poly(amidoxime) ligand (b). | |
To detail investigation of sorption mechanisms, the interaction between metal ions and polymer chelating ligand, the N 1s and O 1s XPS spectra of poly(amidoxime) ligand before and after adsorption of Cu2+ are analysed. The core-level N 1s XPS spectra for both samples are shown in Fig. 14a and b. The N 1s peak of polymer ligand exhibit two peaks at the BEs of 399.0 eV and 399.9 eV, which belong to nitrogen atoms in NH2–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH species, respectively (Fig. 14a). After the adsorption of Cu2+, a new peak at BE of 400.9 eV appeared with the peaks of N 1s (Fig. 14b). These results proved the amidoxime group of polymer ligand is the active group which forms coordinate bonds with Cu2+ after adsorption. The lone pair of electrons in the nitrogen atoms of the amidoxime group is donated to form a coordination bond between Cu2+ and the nitrogen atoms.19,20 The O 1s XPS spectra of polymer ligand also exhibit two peaks at the BEs of 531.0 eV and 532.1 eV, which can be attributed to the oxygen atoms in C–OH and C
N–OH species, respectively (Fig. 14a). After the adsorption of Cu2+, a new peak at BE of 400.9 eV appeared with the peaks of N 1s (Fig. 14b). These results proved the amidoxime group of polymer ligand is the active group which forms coordinate bonds with Cu2+ after adsorption. The lone pair of electrons in the nitrogen atoms of the amidoxime group is donated to form a coordination bond between Cu2+ and the nitrogen atoms.19,20 The O 1s XPS spectra of polymer ligand also exhibit two peaks at the BEs of 531.0 eV and 532.1 eV, which can be attributed to the oxygen atoms in C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH species, respectively (Fig. 14c). After the adsorption of Cu2+, a new peak at BE of 532.9 eV appeared with the peaks of O 1s (Fig. 14d). These results also evidence the oxygen atoms in C
N–OH species, respectively (Fig. 14c). After the adsorption of Cu2+, a new peak at BE of 532.9 eV appeared with the peaks of O 1s (Fig. 14d). These results also evidence the oxygen atoms in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH contribute to the formation of coordinate bond between ligand and metal ions.
N–OH contribute to the formation of coordinate bond between ligand and metal ions.
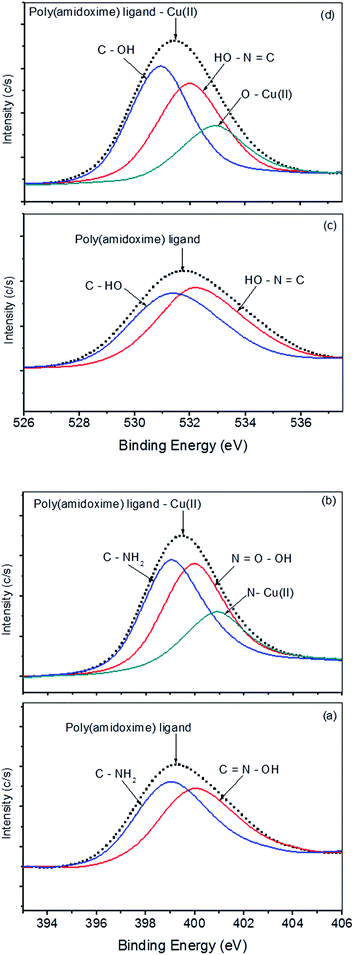 |
| | Fig. 14 N 1s core-level XPS spectra of poly(amidoxime) ligand (a) and poly(amidoxime) ligand with copper (b). O 1s core-level spectra of poly(amidoxime) ligand (c) and poly(amidoxime) ligand with copper (d). | |
It is well documented that when a sorbate is adsorbed on a sorbent through chemical interactions, the chemical state of atoms involved in the sorption process could be changed, resulting in different X-ray photoelectron spectra.21,22 After sorption, the N 1s core-level spectrum showed additional peak at 400.9 eV (Fig. 14b) indicating that N atoms are electron donors during the copper sorption. Since there is a lone pair of electrons in the nitrogen atom, this pair of electrons can be donated to form a coordination bond between a copper species and a nitrogen atom. As a consequence, the electron cloud density of the nitrogen atom is reduced, resulting in higher BE peaks.20 The same analysis is also realistic for the O 1s core-level spectra, with an additional peak at 532.9 eV after sorption (Fig. 14d). Therefore, it can be concluded that the sorption of Cu2+ on the polymer ligand is associated to both nitrogen and oxygen atoms of amidoxime groups.
Elution and reusability studies
The regeneration and reuses of the ligand are important factors for practical applications from the point of view of cost effective.9 We have carried out the elution experiments after removal/sorption test to evaluate the elution and regeneration for reusability of ligand. The release of metal ions from the ligand is possible using acidic condition due to very low sorption at pH 3. Therefore, we can use below pH zero for complete extraction of metal ions from the ligand. Thus, 2 M of HCl solution was used for sufficient to extract the adsorbed Cu2+ ion from the adsorbent. The adsorbent was regenerated into initial form after rinsing with water several times and buffer pH 6 after every elution experiments for reusability of the ligand. The reusability was examined by the sorption/elution process for seven cycles. The sorption process was performed by stirring 150 mg of ligand with 10 mL of 0.1 M Cu2+ solution at pH 6 for 2 h. Desorption study was performed by 2 M HCl solution with 20 mL solution. The sorption/removal and extraction efficiencies was only about 7% decreased after 7 cycles as estimated from Fig. 15. This polymeric ligand can be reused in many cycles without any significant loss of its removal performances. Therefore, the ligand shows high structural stability which promoted the applications of Cu2+ ion removal from environmental wastewater effluents.
 |
| | Fig. 15 Reusability studies of the polymeric ligand in several cycles of sorption/removal – extraction experiments. | |
Discussion
A range of functional or ligand groups in the modified cellulose adsorbent materials contain elements in groups V (nitrogen) and VI (oxygen) of the periodic Table. The ion or molecule possessing a pair of non-bonding electrons which binding metal may be defined as ligand. In this report, a polymeric chelating ligand containing amidoxime ligand was introduced to the metal ions adsorption study. This ligand exhibited high affinity to copper (326.62 mmol g−1) at pH 6 and other metals such as iron, chromium, manganese and cobalt uptake were excellent at pH 6. In comparison with other cellulose-based adsorbents, our prepared ligand from kenaf cellulose showed higher adsorption capacity.
Liu et al.23 synthesized a spheroidal cellulose adsorbent through a grafting reaction using acrylonitrile and converted into the carboxyl groups on its surface. This modified cellulose adsorbent having carboxyl groups was used for the removal of Cu ion from aqueous solutions using a bidentate complexation. Low et al.24 modified wood pulp by esterification reaction to form carboxyl groups of the citric acid and the hydroxyl groups of the wood surface and subsequent adsorption of Cu and Pb ion from aqueous solutions.
A lone pair of electrons on the nitrogen of amino group and this lone pair of electrons can be formed as covalent bond with a metal. For example, an amidoxime groups have a bidentate ligand which loses a proton and a basic lone pair of electrons on the nitrogen to coordinate with the metal ions. O'Connell et al.25 synthesized imidazole by a binding agent on a glycidyl methacrylate grafted cellulose adsorbent and imidazole having a five-membered ring containing two nitrogen atoms. An important aspect of unsaturated nitrogen donors such as imidazole is the possibility of p-back bonding between it and the metal ion.26
It is clearly shown that the significant adsorption capabilities are achieved with the modified cellulose materials. The significant variations in adsorption levels for each cationic species are observed depending on the cellulose modification method and the nature of the chelating or metal binding ligands. The detail mechanism of each adsorption process is difficult to recognize but a number of fundamental interactions are conceivable such as ion exchange, complexation, co-ordination/chelation, electrostatic interactions, acid–base interactions and hydrogen bonding, hydrophobic interactions, physiadsorption and possibly precipitation.27 However, the chemical and physical composition of the modified cellulose, the nature of the metal and solution conditions such as pH, metal concentration and solubility product issues can effects the adsorption interaction.
Cellulose was grafted with the vinyl monomer glycidyl methacrylate using chemical initiating process and cellulose grafted copolymer was further functionalized with thiosemicarbazide for adsorption of Cd2+ and Hg2+ from aqueous solutions.28,29 Authors further extended the cellulose grafted copolymer with β-CD (cyclic oligosaccharides) and quaternary ammonium groups to build cellulose-g-GMA-β-CDN+ adsorbent and the maximum adsorption capacity of Cr6+ reached to 61.05 mg g−1. The adsorption–desorption tests of cellulose derivatives exhibited the good reproducibility of the adsorbent and the adsorbent could be reused.30
The mercaptobenzothiazole adsorbent was prepared from cellulose for the adsorption of Hg2+ with a high adsorption capacity of 204.08 mg g−1 using column method.31 Microwave-induced emulsion copolymerization was conducted for acrylic monomer ethyl acrylate and guar gum. The copolymer sample was used to uptake the cadmium ion and authors claimed that the adsorbent exhibited high reusability and could be successfully recycled.32 A new adsorbent was synthesized from graft copolymerization of glycidyl methacrylate onto zirconium oxide densified cellulose in the presence of N,N′-methylenebisacrylamide as cross-linker having tannin-modified poly(glycidyl methacrylate)-grafted zirconium oxide-densified cellulose.32 The optimum pH for maximum adsorption was found to be 5.5 with the 99.2% removal at an initial concentration of 10 mg L−1. The maximum adsorption capacity was found to be 96.7 mg g−1 for complete removal of Th4+ from simulated seawater. The biopolymer cellulose along with different modifiers/chelating agents have been utilized to study the adsorption of other metal ions such as copper, lead, nickel, etc. A comparison of the adsorption capacities,23–32 shows that kenaf-based amidoxime ligand has high adsorption efficiency for several metal ions as shown in Table 8.
Table 8 Adsorption capacities of some metal ions reported in the literature.17–26
| Adsorbent materials |
Modifier |
Metal ions |
Adsorption capacity (mg g−1) |
| Cellulose |
Sodium hydroxide (carboxyl)23 |
Cu2+ |
70.5 |
| Cellulose |
Glycidyl methacrylate25 |
Cu2+ |
68.5 |
| Cellulose |
Mercaptobenzothiazole30 |
Hg2+ |
204.08 |
| Wood pulp |
Citric acid24 |
Cu2+ |
24.0 |
| Cellulose |
β-CD and quaternary ammonium31 |
Cr6+ |
61.05 |
| Cellulose |
Glycidyl methacrylate32 |
Th4+ |
96.7 |
| Guar gum |
Ethyl acrylate31 |
Cd2+ |
270.27 |
| Chitosan |
Tripolyphosphate27 |
Cu2+ |
200.00 |
| Cellulose |
Poly(amidoxime) ligand (this study) |
Cu2+ (Fe, Cr, co, Ni) |
326.6 (see Table S1) |
Conclusion
A poly(amidoxime) chelating ligand was successfully synthesized from poly(acrylonitrile) grafted kenaf cellulose. The chelation behaviour of the ligand towards some transition metal ions was found to be excellent. The adsorption capacities of the ligand for Cu, Fe, Zn, Ni, Cr ions were observed to be pH-dependent. The rate of equilibrium is very fast, therefore, the column technique would be the most efficient for heavy metals extraction. The low-cost production of kenaf cellulose-based poly(amidoxime) ligand can be considered as an excellent candidate for waste water treatment process since the removal of metal ions was highly efficient. In the present work, we have carried out desorption study which is useful for reusability of polymeric ligand. Real industrial effluent containing heavy metal ions would be removed by this ligand and updated results would be published in future. According to the batch adsorption for isothermal study, transition metal ions sorption onto ligand was well fitted with the Langmuir isotherm model, which suggest that the cellulose based adsorbent surface is homogenous and monolayer property. The sorption/desorption process for seven cycles promising that the new adsorbent can be reused in many cycles without any significant loss in its original removal performances.
Acknowledgements
This research work was supported by the Ministry of Science, Technology and Innovation, Malaysia (RDU 130505).
Notes and references
- S. Kamel, E. M. Hassan and M. El-Sakhawy, J. Appl. Polym. Sci., 2006, 100, 329–334 CrossRef CAS.
- M. R. Lutfor, S. Silong, W. M. Z. Wan Yunus, M. Z. A. Rahman, M. Ahmad and J. Haron, Eur. Polym. J., 2000, 36, 2105–2113 CrossRef CAS.
- A. Bhattacharya and B. N. Misra, Prog. Polym. Sci., 2004, 29, 767–814 CrossRef CAS.
- G. Gurdag, M. Yasar and M. A. Gurkaynak, J. Appl. Polym. Sci., 1997, 66, 929–934 CrossRef CAS.
- E. Wilkins and Q. Yang, J. Environ. Sci. Health, Part A: Environ. Sci. Eng. Toxic Hazard. Subst. Control, 1996, 31, 2111–2128 CrossRef.
- N. V. Narayanan and G. Mahesh, J. Hazard. Mater., 2009, 161, 575–580 CrossRef CAS PubMed.
- W. S. S. Yong, M. R. Lutfor, S. E. Arshad, N. L. Surugau and B. Musta, J. Appl. Polym. Sci., 2012, 124, 4443–4451 CrossRef.
- M. R. Lutfor, S. Silong, W. M. Z. Wan Yunus, M. Z. A. Rahman, M. Ahmad and J. Haron, J. Appl. Polym. Sci., 2001, 79, 1256–1264 CrossRef CAS.
- M. R. Awual, T. Yaita, S. A. El-Safty, H. Shiwaku, H. Suzuki and Y. Okamoto, Chem. Eng. J., 2013, 221, 322–330 CrossRef CAS.
- M. R. Awual, M. M. R. Ismail, T. Yaita, M. A. Khaleque and M. Ferdows, Chem. Eng. J., 2014, 236, 100–109 CrossRef CAS.
- F. L. Chuan, L. R. Jun, X. Feng, J. L. Jina, X. S. Jin and C. S. Run, J. Agric. Food Chem., 2006, 54, 5742–5748 CrossRef PubMed.
- Y. K. Agrawal, Russ. Chem. Rev., 1979, 48, 948–963 CrossRef.
- D. Hall and F. J. Llewellyn, Acta Crystallogr., 1956, 9, 108–112 CrossRef CAS.
- D. W. O'Connell, C. Birkinshaw and T. F. O'Dwyer, J. Chem. Technol. Biotechnol., 2006, 81, 1820–1828 CrossRef.
- M. R. Lutfor, N. N. M. Rohani and M. M. Yusoff, J. Appl. Polym. Sci., 2014, 131, 40833 Search PubMed.
- P. Wang, M. Du, H. Zhu, S. Bao, T. Yang and M. Zou, J. Hazard. Mater., 2015, 286, 533–544 CrossRef CAS PubMed.
- R. S. Norouzian and M. M. Lakouraj, Synth. Met., 2015, 203, 135–148 CrossRef CAS.
- X. Chen, Information, 2015, 6, 14–22 CrossRef.
- Y. Song, J. Li, G. Ye, J. Xu and M. Jiang, Ind. Eng. Chem. Res., 2015, 54, 12367–12373 CrossRef CAS.
- Y. Zhao, X. Wang, J. Li and X. Wang, Polym. Chem., 2015, 6, 5376–5384 RSC.
- J. N. Fiedor, W. D. Bostick, R. J. Jarabek and J. Farrell, Environ. Sci. Technol., 1998, 32, 1466–1473 CrossRef CAS.
- Q. H. Fan, P. Li, Y. F. Chen and W. S. Wu, J. Hazard. Mater., 2011, 192, 1851–1859 CrossRef CAS PubMed.
- M. Liu, Y. Deng, H. Zhan and X. Zhang, J. Appl. Polym. Sci., 2002, 84, 478–485 CrossRef CAS.
- K. S. Low, C. K. Lee and S. M. Mak, Wood Sci. Technol., 2004, 38, 629–640 CrossRef CAS.
- D. W. O'Connell, C. Birkinshaw and T. F. O'Dwyer, J. Appl. Polym. Sci., 2006, 99, 2888–2897 CrossRef.
- D. W. O'Connell, C. Birkinshaw and T. F. O'Dwyer, Bioresour. Technol., 2008, 99, 6709–6724 CrossRef PubMed.
- S. T. Lee, F. L. Mi, Y. J. Shen and S. S. Shyu, Polymer, 2001, 42, 1879–1892 CrossRef CAS.
- Z. Yanmei, J. Qiang, Z. Tianwei and A. Yoshifumi, J. Hazard. Mater., 2011, 187, 303–310 CrossRef PubMed.
- Z. Yanmei, H. Xiaoyi, Z. Min, Z. Xiaofeng and N. Jiangyang, Ind. Eng. Chem. Res., 2013, 52, 876–884 CrossRef.
- K. K. A. Santhana, S. Kalidhasan, R. Vidya and N. Rajesh, Ind. Eng. Chem. Res., 2013, 52, 11838–11849 CrossRef.
- S. Vandana, K. S. Ajit and M. Sadhana, Ind. Eng. Chem. Res., 2009, 48, 4688–4696 CrossRef.
- T. S. Anirudhan and S. R. Rejeena, Ind. Eng. Chem. Res., 2011, 50, 13288–13298 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Photographs of products, TEM image, sensing and colour optimizations by UV spectra. See DOI: 10.1039/c5ra18502e |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water:4
water:4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The product was finally oven dried at 50 °C to a constant weight.7
1). The product was finally oven dried at 50 °C to a constant weight.7


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water; 4
water; 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). About 50% of NaOH solution was added in cold condition and the precipitate form of NaCl was removed using filtration. The pH of the reaction was adjusted to pH 11 by NaOH solution. The ratio of methanol to water was maintained at 4
1). About 50% of NaOH solution was added in cold condition and the precipitate form of NaCl was removed using filtration. The pH of the reaction was adjusted to pH 11 by NaOH solution. The ratio of methanol to water was maintained at 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v). Then poly(acrylonitrile) grafted kenaf cellulose (10 g) was placed into a two-neck round bottom flask fixed with a stirrer, condenser and thermostat water bath.7,8 The prepared hydroxylamine solution was then added to the flask and the reaction was carried out at 70 °C for 6 h. After completion of reaction, the chelating polymeric ligand was separated from hydroxylamine solution by filtration followed by washing with methanolic solution (methanol
1 (v/v). Then poly(acrylonitrile) grafted kenaf cellulose (10 g) was placed into a two-neck round bottom flask fixed with a stirrer, condenser and thermostat water bath.7,8 The prepared hydroxylamine solution was then added to the flask and the reaction was carried out at 70 °C for 6 h. After completion of reaction, the chelating polymeric ligand was separated from hydroxylamine solution by filtration followed by washing with methanolic solution (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water/4
water/4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The ligand was treated with 100 mL of 0.1 M HCl in methanolic solution 5 min for conversion into H-form ligand. The ligand was filtered and washed several times with methanol and dried at 50 °C to a constant weight (see Fig. S1d of ESI†).
1). The ligand was treated with 100 mL of 0.1 M HCl in methanolic solution 5 min for conversion into H-form ligand. The ligand was filtered and washed several times with methanol and dried at 50 °C to a constant weight (see Fig. S1d of ESI†).
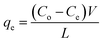

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching and N–H bending modes, respectively (Fig. 1c). In addition, a shoulder created at 3304 cm−1 for both N–H and OH stretching bands and 1401 cm−1 for OH bending was observed (Fig. 1c). It was found that the CN band for 2244 cm−1 disappeared and the new absorption bands for amidoxime group appeared at 3304 cm−1, 1680 cm−1 stretching and 1401 cm−1 bending modes, which the confirmed the of successful synthesis of amidoxime ligand from polyacrylonitrile grafted kenaf cellulose.
N stretching and N–H bending modes, respectively (Fig. 1c). In addition, a shoulder created at 3304 cm−1 for both N–H and OH stretching bands and 1401 cm−1 for OH bending was observed (Fig. 1c). It was found that the CN band for 2244 cm−1 disappeared and the new absorption bands for amidoxime group appeared at 3304 cm−1, 1680 cm−1 stretching and 1401 cm−1 bending modes, which the confirmed the of successful synthesis of amidoxime ligand from polyacrylonitrile grafted kenaf cellulose.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching and N–H bending modes were larger due to the metal–ligand complex ring formation, which is a strong evidence for adsorption occurred by metal ions on the cellulose-based polymeric chelating ligand.
N stretching and N–H bending modes were larger due to the metal–ligand complex ring formation, which is a strong evidence for adsorption occurred by metal ions on the cellulose-based polymeric chelating ligand.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using reaction pH 11 at 70 °C for 4 h. The H-form ligand was obtained after treatment with 0.1 M HCl solution and physical and chemical properties of poly(amidoxime) ligand were summarized in Table 1.
1 using reaction pH 11 at 70 °C for 4 h. The H-form ligand was obtained after treatment with 0.1 M HCl solution and physical and chemical properties of poly(amidoxime) ligand were summarized in Table 1.





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus log
qe versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce. The data were also utilized into Freundlich isotherms model by the plot of log of qe versus Ce as shown in Fig. 12. The KF and n are calculated and presented in Table 6. The Freundlich equation shows that a non-significant correlation (R2 < 0.95) with the experimental data, which suggest that no multilayer adsorption was occurred by selected transition metals. The experimental results of the Freundlich adsorption model with the experimental data were also presented in Table 6.
Ce. The data were also utilized into Freundlich isotherms model by the plot of log of qe versus Ce as shown in Fig. 12. The KF and n are calculated and presented in Table 6. The Freundlich equation shows that a non-significant correlation (R2 < 0.95) with the experimental data, which suggest that no multilayer adsorption was occurred by selected transition metals. The experimental results of the Freundlich adsorption model with the experimental data were also presented in Table 6.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH species, respectively (Fig. 14a). After the adsorption of Cu2+, a new peak at BE of 400.9 eV appeared with the peaks of N 1s (Fig. 14b). These results proved the amidoxime group of polymer ligand is the active group which forms coordinate bonds with Cu2+ after adsorption. The lone pair of electrons in the nitrogen atoms of the amidoxime group is donated to form a coordination bond between Cu2+ and the nitrogen atoms.19,20 The O 1s XPS spectra of polymer ligand also exhibit two peaks at the BEs of 531.0 eV and 532.1 eV, which can be attributed to the oxygen atoms in C–OH and C
N–OH species, respectively (Fig. 14a). After the adsorption of Cu2+, a new peak at BE of 400.9 eV appeared with the peaks of N 1s (Fig. 14b). These results proved the amidoxime group of polymer ligand is the active group which forms coordinate bonds with Cu2+ after adsorption. The lone pair of electrons in the nitrogen atoms of the amidoxime group is donated to form a coordination bond between Cu2+ and the nitrogen atoms.19,20 The O 1s XPS spectra of polymer ligand also exhibit two peaks at the BEs of 531.0 eV and 532.1 eV, which can be attributed to the oxygen atoms in C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH species, respectively (Fig. 14c). After the adsorption of Cu2+, a new peak at BE of 532.9 eV appeared with the peaks of O 1s (Fig. 14d). These results also evidence the oxygen atoms in C
N–OH species, respectively (Fig. 14c). After the adsorption of Cu2+, a new peak at BE of 532.9 eV appeared with the peaks of O 1s (Fig. 14d). These results also evidence the oxygen atoms in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH contribute to the formation of coordinate bond between ligand and metal ions.
N–OH contribute to the formation of coordinate bond between ligand and metal ions.



























