Functional nanoparticle-decorated graphene oxide sheets as stabilizers for Pickering high internal phase emulsions and graphene oxide based foam monoliths†
Yang
Hu
ab,
Jian
Huang
a,
Qi
Zhang
a,
Yu
Yang
a,
Shanshan
Ma
a and
Chaoyang
Wang
 *a
*a
aResearch Institute of Materials Science, South China University of Technology, Guangzhou 510640, China. E-mail: zhywang@scut.edu.cn; Fax: +86-20-22236269; Tel: +86-20-22236269
bInstitute of Biomaterials, College of Materials and Energy, South China Agriculture University, Guangzhou 510642, China
First published on 23rd November 2015
Abstract
Metallic oxide nanoparticle-decorated graphene oxide (GO) sheets were easily fabricated by a redox reaction between GO and metal salts, and then could be used to stabilize oil-in-water (O/W) Pickering high internal phase emulsions (HIPEs). Here, the metallic oxide nanoparticles contained Fe3O4, TiO2, and Mn3O4 nanoparticles. The effects of the concentrations of the metal salt and GO sheets used to prepare the nanoparticle-decorated GO, the internal phase fraction, and the type of oil phase on the formation and properties of HIPEs were systematically investigated. The results showed that the intermediate GO concentration used to prepare the nanoparticle-decorated GO was helpful for the formation of HIPE. And the average diameter of HIPE droplets decreased with the increase in the concentration of metal salt (iron chloride), but enhanced with the increase in the internal phase fraction. Oils with a large viscosity or containing a benzene ring structure were favorable to forming concentrated emulsions with a high internal phase fraction. Furthermore, the nanoparticle-decorated GO stabilized Pickering HIPE was used as a template to prepare functional GO-based porous foams by polymerizing the Pickering HIPE. This research paves a facile and effective way for the fabrication of functional GO-based O/W Pickering HIPEs and porous materials, which may find a wide variety of applications, such as in magnetic, catalytic and electrochemical fields.
Introduction
High internal phase emulsions (HIPEs), also known as gel emulsions which form in either water- or oil-rich regions of water/surfactant/oil systems, have drawn extensive attention as novel reaction media for polymerization in continuous and/or dispersed phases to obtain materials with improved properties such as solid foams1–4 and composites.5,6 The advantages of poly-HIPEs, including facile fabrication processes, tuneable porosity and pore size, make the HIPE-templated materials intelligent enough to satisfy various practical requirements in many domains like food,5 catalysts,7,8 bioseparation,9 adsorption10 and tissue engineering.11 As emulsified systems, HIPEs are commonly defined as type of emulsions characterized by an internal phase fraction larger than 0.74.12 Conventional HIPEs are commonly stabilized with a relatively large amount of small molecule surfactants, which must be only soluble in the continuous phase to prevent emulsion inversion at high internal phase fractions.13 This large quantity of surfactant stores up potential drawback due to its toxicity.14 Moreover, as the surfactants continually and rapidly adsorb and desorb from the interface, the stability of the surfactant-stabilized HIPEs presents an obvious challenge in the practical applications.15A promising alternative is the use of solid particles as emulsifiers to stabilize HIPEs, so-called Pickering HIPEs. The particles are irreversibly adsorbed at the oil/water interface of Pickering HIPEs because of their high attachment energy, which makes the obtained HIPEs extremely stable.16–23 Furthermore, the functional particles used as emulsifiers can endow some new performances to the obtained Pickering HIPEs, for example, Fe3O4 nanoparticles for magnetism,24 TiO2 for catalysis,25 and bovine serum albumin nanoparticles for biocompatibility.26 However, it should be worth noting that only a small number of particles can be served as Pickering HIPE stabilizers. The reported particle emulsifiers include oleic acid modified silica particles,27 PNIPAM-co-MAA microgel particles,28 cellulose nanocrystals,29,30 grafted hydroxyapatite particles31 and lignin particles.32
Graphene oxide (GO) sheet is a product of chemical exfoliation of graphite and has been widely used to produce graphene-based materials, which have been hotspots in electrochemistry,33–35 biology,36,37 physics38 and energy science.39,40 GO has been known to disperse well in water for the ionizable edge –COOH groups and phenol hydroxyl groups on the basal plane.41 Combined with hydrophobic polyaromatic island of unoxidized benzene rings of its basal plane, GO should be viewed as an amphiphilic substance, which is able to adsorb on interfaces and lower the surface or interfacial tension.42 Several attempts based on this property have been conducted to use GO as colloidal surfactants. GO can emulsify oil into water to form Pickering emulsions.43–48 However, the obtained GO-stabilized emulsions are mostly diluted emulsions, little literature involves the formation of GO-stabilized oil-in-water (O/W) HIPEs. It should be ascribed to the reason that the excess hydrophilicity and high negative charge make GO not effectively prevent emulsion droplets from coalescence in a very concentrated state. Fortunately, surface modification of GO sheets is a promising strategy to overcome the above issue.
In this work, GO sheets are decorated by the in situ deposition of metal oxide nanoparticles including Fe3O4, TiO2 and Mn3O4 on their surfaces. And the nanoparticle-decorated GO sheets possess suitable wettability and can be used to stabilize O/W Pickering HIPEs with IPF more than 90%. The HIPEs stabilized by nanoparticle-modified GO sheets exhibit excellent stability with a shelf-life more than 6 months. Thus, the nanoparticle-decorated GO stabilized HIPEs are ideal templates for fabricating porous GO-based materials with some functions, which can be adjusted by anchoring various nanoparticles on the GO sheets. This investigation provides guidance for the preparation of functional O/W Pickering HIPEs and porous GO-based materials, which may find wide range of applications, such as in magnetic, catalytic and electrochemical fields.
Experimental methods
Materials
Graphite, dodecane and n-hexane were obtained from J&K Chemical Ltd., China. Potassium permanganate, iron chloride tetrahydrate, toluene, hydrogen peroxide (30%), formaldehyde aqueous solution (37%), hydrochloric acid (37%), ammonia aqueous solution (25%), diethylenetriamine (DETA), concentrated sulfuric acid, petroleum ether and ethanol were bought from Guangzhou Chemical Factory, China. Ethylenediamine (EDA), liquid paraffin and acetic acid were purchased from Jiangsu Qiangsheng Chemical Co. Ltd., China. Triethanolamine (TEA), aqueous titanium trichloride and manganese chloride (MnCl2) were provided by Tianjin Fuchen Chemical Reagent Co. Ltd., China. Melamine was supplied by the Sinopharm Chemical Reagent Co. Ltd., China. All the chemicals were used as received without purification. Water used in all experiments was produced by filtration and deionization using a Millipore purification apparatus (resistivity more than 18.0 MΩ cm).Fabrication of Fe3O4 nanoparticle-decorated GO sheets
GO sheets were fabricated from purified natural graphite using a modified Hummers method,49 and the detailed experimental procedures could be found in our previous report.50 The obtained GO was freeze-dried, and then was dispersed in water by ultrasonication for 1 h to form GO aqueous suspension (3 mg mL−1).The Fe3O4 nanoparticle-decorated GO sheets: 2 mL of the as-prepared GO aqueous suspension was adjusted to pH 11.5 by adding EDA. Then, 50 mg of iron chloride tetrahydrate (FeCl2) was added into the GO aqueous suspension, and the system was stirred evenly at room temperature. After 5 min, the Fe3O4 nanoparticle-decorated GO sheet aqueous suspension was formed. For further characterization, the prepared nanoparticle-decorated GO suspensions were centrifugally washed five times with water, followed by freeze-drying to obtain the dried nanoparticle-decorated GO.
Preparation of Pickering HIPEs stabilized by nanoparticle-decorated GO sheets
The Pickering HIPEs were normally prepared by vigorously hand-shaking while slowly adding the internal oil phase to the as-prepared nanoparticle-decorated GO suspension. After all of the oil phase had been added, the mixture was hand-shaken for another 3 min to generate viscous and homogeneous HIPEs. In this study, the experimental factors such as the concentrations of metal salt and GO used to prepare the nanoparticle-decorated GO, the kind of oils and the internal phase volume fraction were varied in order to discuss their effects on the formation and properties of HIPEs.Preparation of porous nanoparticle-modified GO/poly(melamine formaldehyde) (PMF) foam
Firstly, the melamine formaldehyde pre-polymer (pre-MF) was synthesized by the procedures described in our previous study.32 Then, the continuous aqueous phase was prepared by mixing 0.5 mL of pre-MF solution and 1.5 mL of nanoparticle-modified GO aqueous suspension, and the pH was adjusted to acidic range by adding acetic acid. Thereafter, the Pickering HIPE was prepared by vigorously hand-shaking while slowly adding 8 mL of dodecane acted as oil phase to the aqueous phase. Once all of dodecane had been added, the HIPE was obtained by hand-shaking for another 3 min, then transferred into an oven and polymerized for 4 h at 60 °C. Furthermore, the resulting poly-HIPE was immersed into petroleum ether to remove dodecane, and then washed with ethanol. Finally, porous nanoparticle-modified GO/PMF foam was obtained after being dried in an oven at 50 °C for 24 h.Characterization
The morphology of the GO and nanoparticle-decorated GO sheet was observed by a JEM-2010HR transmission electron microscope (TEM, JEOL Ltd., Japan) under accelerating voltage of 200 kV. Fourier transform spectrometer infrared (FTIR) spectra of the samples were tested on a German Vector-33 IR instrument using KBr pellets. The zeta potentials and diameters of nanoparticle-modified GO suspensions prepared at different amounts of metal salt were measured using a Malvern Zeta Sizer Nano ZS90 at 25 °C. Thermogravimetry analysis (TGA) of the samples was performed with a TG209 thermo-analyzer in air from 40 to 800 °C at a heating rate of 10 °C min−1. X-ray diffraction (XRD) patterns of the samples were obtained using an X'pert PRO diffractometer fitted with a Cu Kα radiation source at room temperature. Magnetic property of the samples was analyzed with a physical property measurement system (PPMS, PPMS-9, Quantum Design INC., USA). The optical micrographs of Pickering HIPEs placed on glass slides were taken using an optical microscope (Carl Zeiss, German) fitted with a Nikon COOLPIX 4500 digital camera. The photographs of Pickering HIPEs were captured by digital camera (Sony W390). The average diameter and diameter distribution of the emulsion droplets were measured using a Malvern Mastersizer 2000. The confocal micrographs of Pickering HIPE was obtained using a Leica TCS-SP2 confocal laser scanning microscope (CLSM) with a 10× objective with a numerical aperture of 1.4. The pH value was monitored using a PHB-2 pH meter (Leici, China). The morphologies of porous modified GO/PMF foams were observed using a Zeiss EVO 18 scanning electron microscope (SEM) fitted with an X-ray energy dispersive spectrometer (EDS) at 10 kV acceleration voltage.Results and discussion
Preparation of Fe3O4 nanoparticle-decorated GO sheets
Nano/microparticle interfacial self-assembly to form Pickering emulsion droplets has been well documented in the recent years. Generally, particles with an oil–water contact angle slightly less than 90° would stabilize O/W emulsions and those with the contact angle slightly greater than 90° would stabilize water-in-oil (W/O) emulsions, but particles with the contact angle that deviates greatly from 90° (very hydrophobic or very hydrophilic) are not observed to stabilize emulsions.51 In the part of O/W emulsion systems, the emulsifying ability of particles is related with their zeta potentials. The particles with abundant surface charges wouldn't be able to stabilize oil droplets, as the strong electrostatic interaction between particles resulted in an incomplete particle cover on the oil droplet surface, which wouldn't effectively promote the emulsion stability during the terms of flocculation, creaming and sedimentation.18–21The GO sheets were covered with abundant oxygen-containing groups by the strong chemical oxidation in the preparation process. Therefore, the GO sheets were hydrophilic, and had a high negative charge (zeta potential ca. −60 mV), which resulted in the insufficient interfacial activity of GO sheets to stabilize emulsion droplets. To solve this issue, some attempts through adjusting the pH value of aqueous phase or modifying GO sheets with surfactants had successfully prepared GO-stabilized O/W Pickering emulsions.52,53 However, the emulsions became to breakdown when the internal phase volume fraction was larger than 74%. It could be ascribed to a large amount of negative charges on the GO surface, which led to an incomplete particle cover layer on the droplets surface. The resultant surplus gap among the GO sheets provided channels for oil diffusion among neighbour HIPE droplets, and in turn emulsion droplet coalescence happened. On the basis of this point of view, reducing the charge of GO sheets was highly beneficial for forming GO-stabilized Pickering HIPE systems without any surfactants.
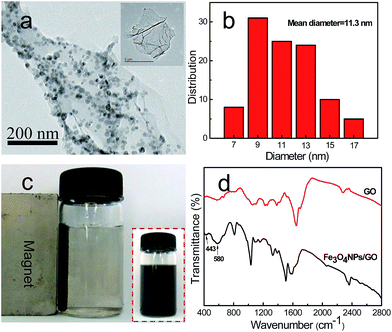
In this study, we explored the in situ generation of metallic oxide nanoparticles on the GO surface to efficiently reduce the negative charges on the GO sheet surface. Several nanoparticles including Fe3O4, TiO2, and Mn3O4 were decorated on GO sheets. The synthesis of Fe3O4 nanoparticle-decorated GO sheets was presented as a typical example (Fig. 1). Herein, we used EDA to adjust the pH value of GO aqueous suspension to 11.5. Noting that EDA not only served as pH modifier, but also acted as reducing agent, which could react with the hydroxyl and carboxyl groups on the GO surface to slightly reduce GO. The zeta potential of EDA-modified GO decreased to ca. −40 mV. Subsequently, FeCl2 was mixed with the EDA-modified GO sheet suspension. The Fe2+ ions originated from FeCl2 were absorbed onto GO surface through electrostatic attraction, and oxidized to form Fe3O4 nanoparticle-decorated GO by the spontaneous in situ deposition of Fe3O4 nanoparticles onto the reduced GO surface.54 Fig. 2a shows the TEM image of Fe3O4 nanoparticle-decorated GO sample. It was observed that the spherical Fe3O4 nanoparticles were independently anchored onto the GO sheet surface. And the average diameter of Fe3O4 nanoparticles was about 11.3 nm (Fig. 2b), which was estimated by measuring the particles from the TEM image using Nano Measurer 1.2 software.
As shown in Fig. 2c, the Fe3O4 nanoparticle-decorated GO sheets were well dispersed in water to form a stable black suspension. And the modified GO sheets could be attracted by an external magnet, indicating that these modified GO sheets exhibited a clear magnetic response. This result confirmed that the magnetic Fe3O4 nanoparticles were deposited on the GO sheets, which had imparted magnetic properties to the GO sheets. And the magnetization curve of Fe3O4 nanoparticle-decorated GO showed that the saturation magnetization value of Fe3O4 nanoparticle-decorated GO was 34.99 emu g−1 (Fig. S1†). The EDS spectrum of Fe3O4 nanoparticle-decorated GO (Fig. S2a†) showed that the major elements of the modified GO consisted of carbon (C), oxygen (O), and iron (Fe). FTIR was also used to characterize the composition of GO sheets before and after being modified. As shown in Fig. 2d, compared with the FTIR spectrum of GO sheets, the FTIR spectrum of Fe3O4 nanoparticle-decorated GO sheets had two new peaks at about 443 cm−1 and 580 cm−1, which corresponded to the characteristic peaks of Fe3O4. This suggested that Fe2+ ions had been oxidized into Fe3O4 nanoparticles by GO as an efficient oxidizing reagent and thus presented as Fe3O4 nanoparticles on the GO sheet surface. Furthermore, the XRD pattern of Fe3O4 nanoparticle-decorated GO (Fig. S2b†) demonstrated that the characteristic diffraction peaks of Fe3O4 derived from the (220), (311), (400), (422), (511) and (440) planes. These above results suggested that Fe3O4 nanoparticle-decorated GO had been successfully fabricated. The weight of Fe3O4 in the Fe3O4 nanoparticle-decorated GO sheets was determined using TGA (Fig. S2c†), and the result showed that the weight percentage of Fe3O4 nanoparticles was about 50.9%.
The zeta potential and size of the obtained Fe3O4 nanoparticle-decorated GO sheets could be tuned easily by adjusting the concentration of FeCl2. As shown in Fig. 3, when the concentration of GO was fixed at 3 mg mL−1, the negative charges on the Fe3O4 nanoparticle-decorated GO surface reduced gradually with increasing the concentration of FeCl2. With further increasing the concentration of FeCl2, the zeta potential of Fe3O4 nanoparticle-decorated GO sheets became positive until a platform appeared around +18 mV. The above results indicated that the addition of FeCl2 could indeed change the charge amount on GO sheet surface. Furthermore, the size of Fe3O4 nanoparticle-decorated GO sheets varied with the change of the zeta potential value. There was a maximum size of ca. 1.7 µm at the zeta potential of around 0 mV. The cover density of Fe3O4 nanoparticles on the GO surface should be increased with increasing the concentration of FeCl2, which neutralized the more negative charges on the GO sheet surfaces. Meanwhile, the size of Fe3O4 nanoparticle-decorated GO sheets increased with the decrease of the surface charge density because of Fe3O4 nanoparticle-decorated GO aggregation under the weak electrostatic repulsion.
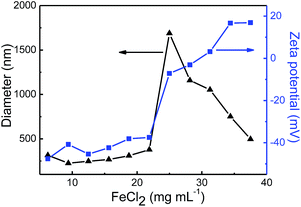 | ||
| Fig. 3 Sizes and zeta potential values of Fe3O4 nanoparticle-decorated GO sheets prepared at different FeCl2 concentrations. | ||
Formation of Pickering HIPEs
The Fe3O4 nanoparticle-decorated GO sheets were introduced to form O/W Pickering HIPEs as shown in Fig. 4. The toluene acted as oil phase was gradually added to the Fe3O4 nanoparticle-decorated GO sheet aqueous suspension (aqueous phase) under handshaking. The modified GO sheets assembled at the oil–water interface to effectively stabilize black viscous Pickering emulsions. The type of the prepared emulsion was determined by confocal laser scanning microscopy and the droplet test (Fig. S3†). For CLSM observation, the aqueous phase was stained with rhodamine B, which was green in the CLSM image. It was found that aqueous phase acted as the continuous phase and the oil phase was the disperse phase (Fig. S3a†). In the drop test, the emulsion collapsed immediately in water, while the emulsion floated on the surface of toluene (Fig. S3b†), which also indicated that the continuous phase was the aqueous phase and the oil phase was the disperse phase. In short, the above results showed that the HIPE stabilized by Fe3O4 nanoparticle-decorated GO sheets was an O/W type emulsion. The zeta potential of particles has an obvious influence on the formation of the Pickering HIPEs. As above mentioned, the zeta potential of the modified GO sheets could be tuned by varying the concentration of FeCl2. Thus, the HIPEs stabilized by Fe3O4 nanoparticle-decorated GO sheets prepared from different concentration of FeCl2 were investigated as shown in Fig. 5. Four types of Fe3O4 nanoparticle-decorated GO sheets fabricated at 18.75, 25, 31.25 and 37.5 mg mL−1 of FeCl2 were used to prepare Pickering HIPEs. The zeta potentials of the corresponding Fe3O4 nanoparticle-decorated GO sheets were −38.1, −7.15, +3.16 and +16.9 mV, respectively. From Fig. 5, it was observed that all four modified GO sheets were successfully used to stabilize O/W Pickering HIPEs. The emulsion droplets were compact with a little deformation, appearing as a typical Pickering HIPE. The size distributions of all HIPE droplets were polydisperse, and the average diameters of the droplets ranged from 150.8 to 89.2 µm (see Fig. S4†). It was found that increasing the concentration of FeCl2 in the GO modification process led to the decrease in the average droplet diameter. Thus, the Pickering HIPEs with different diameters could be obtained by tuning the concentration of FeCl2. | ||
| Fig. 4 Schematic illustration of the fabrication of O/W Pickering HIPEs stabilized by Fe3O4 nanoparticle-decorated GO sheets. | ||
A series of parallel tests were conducted to systematically investigate the effect of the preparation parameters on the formation of toluene-in-water HIPEs stabilized with Fe3O4 nanoparticle-decorated GO sheets (Table S1†). Firstly, the GO sheet concentration was fixed at 3 mg mL−1, stable HIPEs could be formed when the added FeCl2 amount was more than 12.5 mg. Then, the FeCl2 concentration was fixed at 12.5 mg mL−1, only the GO concentration of 3 mg mL−1 could be used to form stable HIPE. Moreover, the relationship of the GO and FeCl2 concentrations with the maximum internal phase fractions of the toluene-in-water Pickering emulsions was systematically summarized in Fig. S5.† It revealed that the GO concentration used to stabilize Pickering HIPEs should be moderate. If the GO concentration was too low (1 mg mL−1), there was not enough modified GO sheets to cover the O/W interface and stabilize the oil droplets. If the GO concentration was too high (6 mg mL−1), excess GO sheets could aggregate to form large GO sheets in the aqueous phase, which could not be effectively absorbed at the O/W interface because of the requirement of high interfacial energy. Thus, both the too low and high GO concentration failed to prepare the HIPEs. When the GO concentration ranged from 2 to 5 mg mL−1, the stable HIPEs could be formed, and the maximum internal phase fractions were able to be adjusted by varying the concentration of FeCl2. Furthermore, directly using the mixture of Fe3O4 nanoparticles (ca. 10 nm in diameter) and GO sheets, the HIPE could not be formed. This result was demonstrated that immobilizing Fe3O4 nanoparticles on the GO sheet surface was necessary to form stable Pickering HIPEs, and simply physical blending these two nano-materials couldn't effectively prevent emulsion droplet coalescence. This phenomenon should be due to the fact that the anchoring of Fe3O4 nanoparticles on GO sheet surface not only screened redundant negative charges on the GO sheet surface, but also resulted in the modified GO sheets with very rough surface, which assisted in the stability of Pickering emulsions.55 In addition, using other pH modifiers like DETA, sodium hydroxide (NaOH), ammonia aqueous solution (NH3·H2O), no HIPEs were formed. This could be ascribed to the fact that EDA in the system not just acted as the pH modifier, but also served as a reducing agent to bridge between GO and Fe3O4 nanoparticles. These Fe3O4 nanoparticle-decorated GO sheets used EDA as pH modifier could tightly absorb on the surface of emulsion droplets like an octopus, providing a more compact barrier against droplet coalescence, which was helpful to form stable HIPEs.
Different oils were further considered to be emulsified with the Fe3O4 nanoparticle-decorated GO sheet aqueous suspension. The maximum internal phase fractions of the resultant emulsions are shown in Fig. 6. It was observed that all the oils could be emulsified by the Fe3O4 nanoparticle-decorated GO sheets, but not all of them could be used to generate HIPEs. Furthermore, it was found that the oils with large viscosity or containing a benzene ring structure could enhance the emulsion stability and be beneficial to forming very concentrated emulsions. The results might be ascribed to these facts that the large viscosity of oil restricted the oil diffusion among the neighbouring emulsion droplets, and the benzene ring structure of oil could interact with GO sheets by the π–π interaction effects to assist with the absorption of the modified GO sheets on the O/W interfaces.
The microstructure of the obtained HIPEs was closely associated with the internal phase fractions. Herein, dodecane was used as oil phase to prepare dodecane-in-water Pickering HIPEs stabilized by Fe3O4 nanoparticle-decorated GO sheets with different internal phase fractions. The optical microscopic images of HIPEs with internal phase fractions of 75, 80 and 85 vol% are presented in Fig. 7. All the droplets squeezed together and exhibited polydispersity. In addition, with the increase of internal phase fraction, the shape of emulsion droplets changed from spherical to polyhedral, and the average diameters of the emulsion droplets increased accordingly from 102.5 to 134.6 µm, which was consistent with the trend previously reported in other Pickering HIPE system.56 The result was attributed to the fact that with the increase of internal phase fraction, the fixed concentration of Fe3O4 nanoparticle-decorated GO sheets became insufficient to cover the enhanced oil/water interfacial areas, which resulted in the destabilization of Pickering HIPEs.
It was well-known that the excellent stability was a major dominance of Pickering HIPEs compared with conventional surfactant-stabilized HIPEs.15,17 The stability of Fe3O4 nanoparticle-modified GO-based Pickering HIPE was further tested. The stable emulsion could be stored for 6 months without phase separation. Fig. 8 shows the optical microscopic image and diameter distribution of the emulsion presented in Fig. 5a after standing for 6 months. It was observed that some aggregations of GO sheets were dispersed in the aqueous phase, and the average diameter of HIPE droplets decreased from 150.8 to 74.7 µm. The results might be attributed to the collapse of some big emulsion droplets and the volatilization of the inner toluene phase. Nevertheless, the emulsion after standing for 6 months indeed maintained the morphology of HIPE droplets with spherical shape and dense packing, showing the good stability of Pickering HIPEs stabilized with Fe3O4 nanoparticle-decorated GO sheets.
 | ||
| Fig. 8 Optical microscopic image (a) and size distribution (b) of the emulsion presented in Fig. 5a after standing for 6 months. The inset is the digital photograph of the corresponding emulsion. The scale bar is 100 µm. | ||
HIPEs stabilized by various nanoparticle-decorated GO sheets
Recently, graphene-based materials had received intense attention in electrochemical applications like supercapacitors, oxygen reduction reactions, and capacitive deionization.57 Incorporation of metal oxides would remarkably improve the functionality of graphene-based materials.57–62 As mentioned before, one of the advantages of the Pickering HIPE templates compared with surfactant-stabilized HIPEs is that some functional properties can be simply taken into the templates by using the corresponding functional particles as the Pickering emulsifiers. As the successful fabrication of the Pickering HIPE stabilized with Fe3O4 nanoparticle-decorated GO sheets, the other nanoparticle-decorated GO sheets were further used for preparing other Pickering HIPEs to explore the nanoparticle-modified GO sheets as effective and general particulate emulsifiers for preparing functional Pickering HIPEs. Herein, more types of metal oxide nanoparticle-decorated GO sheets (including TiO2 and Mn3O4) had been synthesised and introduced to prepare the Pickering HIPEs. TEM studies of TiO2 and Mn3O4 nanoparticle-decorated GO samples (Fig. S6†) indicated that the spherical nanoparticles were anchored onto the GO sheet surfaces, and the average diameters of TiO2 and Mn3O4 nanoparticles were about 9.6 nm and 13.1 nm, respectively. In addition, the XRD researches of TiO2 and Mn3O4 nanoparticle-decorated GO (Fig. S7a†) showed that the characteristic diffraction peaks of TiO2 derived from the (101), (004), (200), (105), (211) and (204) planes, and the characteristic diffraction peaks of Mn3O4 derived from the (112), (103), (211), (220), (105), (312) and (321) planes, respectively. This result indicated that TiO2 and Mn3O4 nanoparticle-decorated GO had been successfully synthesised. And the TGA test results (Fig. S7b†) showed that the weight percentages of TiO2 and Mn3O4 in the related nanoparticle-decorated GO sheets were about 34.0% and 39.6%, respectively. As expected, both of TiO2 and Mn3O4 nanoparticle-decorated GO sheets could be used to successfully stabilize dodecane-in-water Pickering HIPEs. The typical optical microscopy images are shown in Fig. 9a and b. It was observed that the Pickering HIPE droplets were spherical and packed, and their diameters were polydisperse. Furthermore, several oils were further used to be emulsified with these two modified GO sheet aqueous suspension to study the maximum internal phase fractions of the relevant Pickering emulsions. It was found that like the Fe3O4 nanoparticle-decorated GO sheets, only the oil phases with large viscosity or containing benzene ring structures, such as styrene, liquid paraffin, hexadecane and dodecane, could be emulsified with TiO2 or Mn3O4 nanoparticle-decorated GO sheet aqueous suspension to form HIPEs (Fig. 9c and d). The above results showed that the nanoparticle modification method has a general applicability in preparing functional GO sheet-stabilized O/W Pickering HIPEs. In addition, the O/W Pickering HIPEs stabilized with TiO2 and Mn3O4 nanoparticle-decorated GO sheets were very stable with a little phase separation after standing for 6 months. In short, it was concluded that the O/W Pickering HIPEs stabilized with nanoparticle-decorated GO sheets should be very useful as templates for fabricating the functional GO-based nanocomposite (NC) porous materials with well-defined structures and unique functional property such as electrochemistry, magnetism and catalysis properties.Preparation of Fe3O4 nanoparticle-decorated GO/PMF foam
The O/W HIPE stabilized with Fe3O4 nanoparticle-decorated GO sheets and containing the pre-MF monomers in aqueous phase was sealed in a glass bottle and then transferred into an oven. The pre-MF monomers were polymerized at 60 °C for 4 h. The product was purified with petroleum ether and ethanol to remove dodecane and water. And the porous nanoparticle-decorated GO/PMF foam was obtained by air-drying at room at 50 °C for 24 h (Fig. S8†). It was observed that the obtained NC foam demonstrated good structural integrity and mechanical stability. Then the foams froze by liquid nitrogen were sliced using a razor blade, and the inner pore structure of NC foam was observed by SEM. The corresponding SEM images are presented in Fig. 10, showing that the porous NC foam with well-defined pore structure is successfully fabricated by templating O/W Pickering HIPE. As observed, the NC foam exhibited a highly closed-cell structure with no pores interconnected by pore throats, and the average pore size was approximately 100 µm. In fact, previous researches had reported that the polymerization of Pickering HIPEs usually resulted in a closed-cell porous material.63 It was quite reasonable that the closed-cell porous structure was presented because Pickering emulsion was greatly stable, and the solid particulate emulsifiers at the interface acted like a steric barrier against collapse. And the NC foam was examined by EDS to assess the compositions on the pore wall surface. EDS results of NC foam (Fig. S9a†) showed that the main elements were carbon (C), nitrogen (N), oxygen (O) and iron (Fe). As we known that the Fe element was derived from Fe3O4 nanoparticle-decorated GO, which confirmed the embedding of Fe3O4 nanoparticle-decorated GO on the foam pore wall. FTIR spectrum of the NC foam (Fig. S9b†) displayed the Fe–O vibration peak of Fe3O4 at 580 cm−1, which indicated the presence of Fe3O4 nanoparticle-decorated GO in the foam matrix. Furthermore, the XRD study of NC foam (Fig. S9c†) showed the characteristic peaks of Fe3O4 derived from the (220), (311), (400), (511) and (440) planes, implying the existence of Fe3O4 nanoparticle-decorated GO in the NC foam. According to the results of the EDS, FTIR and XRD analysis, the Fe3O4 nanoparticle-decorated GO based NC foam was successfully synthesised. Because of the magnetism of Fe3O4 nanoparticles, the NC foam containing Fe3O4 nanoparticle-decorated GO should be magnetic. Herein, the magnetization of the NC foam was investigated as a function of the applied magnetic field. The result indicated that the NC foam was magnetic, and its saturation magnetization value was 0.97 emu g−1 (Fig. S1†). In this case, it could be considered that the saturation magnetization of the NC foam depended mainly on the content of the Fe3O4 nanoparticles in the NC foam. The weight percentage of Fe3O4 nanoparticles in the NC foam was only about 2.9% (Fig. S9d†), thus the saturation magnetization value for the NC foam was obviously lower than that for Fe3O4 nanoparticles of 67.1 emu g−1.64 According to these above results, we proposed a facile and effective strategy to prepare variety of macroporous functional GO-based composite foams for wide ranges of applications. | ||
| Fig. 10 SEM images of porous Fe3O4 nanoparticle-decorated GO/PMF foam prepared from HIPE in different magnifications. | ||
Conclusions
In summary, metallic oxide nanoparticle-decorated GO sheets were facially prepared by a redox reaction to in situ generate metallic oxide nanoparticle on the GO sheet surface. The nanoparticles deposited on the GO sheet surface included Fe3O4, TiO2, and Mn3O4 nanoparticles. The resulting nanoparticle-decorated GO sheets could be used to stabilize O/W Pickering HIPEs. The obtained Pickering HIPEs were considered as an ideal template for the fabrication of multifunctional GO-based porous materials with both the pristine GO characteristics and the functionalities of the nanoparticle components. This research provides a new method to prepare functional GO-based Pickering HIPEs and porous hybrid materials with novel structures, which may be suitable for a wide variety of applications including in magnetic, catalytic and electrochemical fields.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21274046 and 21474032), the National Natural Basic Research Program of China (973 Program, 2012CB821500), the Natural Science Foundation of Guangdong Province (S2012020011057) and the Fundamental Research Funds for the Central Universities (D2154820). Yang Hu and Jian Huang contributed equally to this work.References
- R. Mezzenga, J. Ruokolainen, G. Fredrickson, E. J. Kramer, D. Moses, A. J. Heeger and O. Ikkala, Science, 2003, 299, 1872 CrossRef CAS PubMed.
- A. Fameau, S. Lam and O. D. Velev, Chem. Sci., 2013, 4, 3874 RSC.
- Z. F. Li, T. Ming, J. F. Wang and T. Ngai, Angew. Chem., Int. Ed., 2009, 48, 8490 CrossRef CAS PubMed.
- D. W. Johnson, C. Sherborne, M. P. Disbury, C. Pateman, N. R. Cameron and F. Claeyssens, Adv. Mater., 2013, 25, 3178 CrossRef CAS PubMed.
- G. Calderó, A. Patti, M. Llinàs and M. J. García-Celma, Curr. Opin. Colloid Interface Sci., 2012, 17, 255 CrossRef.
- S. W. Zou, Z. J. Wei, Y. Hu, Y. H. Deng, Z. Tong and C. Y. Wang, Polym. Chem., 2014, 5, 4227 RSC.
- H. P. Gao, Y. X. Peng, J. M. Pan, J. Zeng, C. H. Song, Y. L. Zhang, Y. S. Yan and W. D. Shi, RSC Adv., 2014, 4, 43029 RSC.
- F. Y. Yi, F. G. Xu, Y. Gao, H. M. Li and D. Y. Chen, RSC Adv., 2015, 5, 40227 RSC.
- F. Audouin, R. Larragy, M. Fox, B. O'Connor and A. Heise, Biomacromolecules, 2012, 13, 3787 CrossRef CAS PubMed.
- H. Liu and C. Y. Wang, RSC Adv., 2014, 4, 3864 RSC.
- E. M. Christenson, W. Soodi, J. L. Holm, N. R. Carmeron and A. G. Mikos, Biomacromolecules, 2007, 8, 3806 CrossRef CAS PubMed.
- M. S. Silverstein, Prog. Polym. Sci., 2014, 39, 199 CrossRef CAS.
- G. Q. Sun, Z. F. Li and T. Ngai, Angew. Chem., Int. Ed., 2010, 49, 2163 CrossRef CAS PubMed.
- P. Viswanathan, S. Chirasatitsin, K. Ngamkham, A. J. Engler and G. Battaglia, J. Am. Chem. Soc., 2012, 134, 20103 CrossRef CAS PubMed.
- B. P. Binks, Curr. Opin. Colloid Interface Sci., 2002, 7, 21 CrossRef CAS.
- Y. Hu, H. C. Gao, Z. S. Du, Y. X. Liu, Y. Yang and C. Y. Wang, J. Mater. Chem. B, 2015, 3, 3848 RSC.
- B. P. Binks and R. Murakami, Nat. Mater., 2006, 5, 865 CrossRef CAS PubMed.
- A. R. Morgan, N. Ballard, L. A. Rochford, G. Nurumbetov, T. S. Skelhon and S. A. F. Bon, Soft Matter, 2013, 9, 487 RSC.
- T. Chen, P. J. Colver and S. A. F. Bon, Adv. Mater., 2007, 19, 2286 CrossRef CAS.
- Z. Zheng, X. H. Zheng, H. T. Wang and Q. G. Du, ACS Appl. Mater. Interfaces, 2013, 5, 7974 CAS.
- M. Hermes and P. S. Clegg, Soft Matter, 2013, 9, 7568 RSC.
- N. Byrne, R. DeSilva, C. P. Whitby and X. G. Wang, RSC Adv., 2013, 3, 24025 RSC.
- D. Y. Zang and P. S. Clegg, Soft Matter, 2013, 9, 7048 Search PubMed.
- T. T. Li, H. R. Liu, L. Zeng, S. Yang, Z. C. Li, J. D. Zhang and X. T. Zhou, J. Mater. Chem., 2011, 21, 12865 RSC.
- A. Menner, V. Ikem, M. Salqueiro, M. S. P. Shaffer and A. Bismarck, Chem. Commun., 2007, 4274 RSC.
- Z. F. Li, M. Xiao, J. F. Wang and T. Ngai, Macromol. Rapid Commun., 2013, 34, 169 CrossRef PubMed.
- V. Ikem, A. Menner, T. S. Horozov and A. Bismarck, Adv. Mater., 2010, 22, 3588 CrossRef CAS PubMed.
- Z. F. Li, X. L. Wei and T. Ngai, Chem. Commun., 2011, 47, 331 RSC.
- I. Capron and B. Cathala, Biomacromolecules, 2013, 14, 291 CrossRef CAS PubMed.
- K. Y. Lee, J. J. Blaker, R. Murakami, J. Y. Y. Heng and A. Bismarck, Langmuir, 2014, 30, 452 CrossRef CAS PubMed.
- Y. Hu, Y. Yang, M. Hu, X. Y. Gu and C. Y. Wang, J. Controlled Release, 2015, 213, e127 CrossRef.
- Y. Yang, Z. J. Wei, C. Y. Wang and Z. Tong, Chem. Commun., 2013, 49, 7144 RSC.
- S. J. Guo and S. J. Dong, Chem. Soc. Rev., 2011, 40, 2644 RSC.
- Q. Liu, Q. W. Shi, H. Z. Wang, Q. H. Zhang and Y. G. Li, RSC Adv., 2015, 5, 47074 RSC.
- X. Y. Gu, Y. Yang, Y. Hu, M. Hu, J. Huang and C. Y. Wang, RSC Adv., 2014, 4, 63189 RSC.
- R. R. Nair, P. Blake, A. N. Grigorenko, K. S. Novoselov, T. J. Booth, T. Stauber, N. M. R. Peres and A. K. Geim, Science, 2008, 320, 5881 CrossRef PubMed.
- L. M. Saeed, M. Mahmood, Y. Xu, Z. A. Nima, G. K. Kannarpady, S. M. Bratton, E. Dervishi, D. Casciano, S. Ali, P. A. Crooks, A. Radominska-Pandya and A. S. Biris, RSC Adv., 2015, 5, 44022 RSC.
- S. Pisana, M. Lazzeri, C. Casiraghi, K. S. Novoselov, A. K. Geim, A. C. Ferrari and F. Mauri, Nat. Mater., 2007, 6, 198 CrossRef CAS PubMed.
- Q. Y. He, S. X. Wu, Z. Y. Yin and H. Zhang, Chem. Sci., 2012, 3, 1764 RSC.
- H. J. Zhang, L. J. Gao and S. B. Yang, RSC Adv., 2015, 5, 43798 RSC.
- L. J. Cote, J. Kim, Z. Zhang, C. Sun and J. X. Huang, Soft Matter, 2010, 6, 6096 RSC.
- J. Kim, L. J. Cote, F. Kim, W. Yuan, K. R. Shull and J. X. Huang, J. Am. Chem. Soc., 2010, 132, 8180 CrossRef CAS PubMed.
- Z. W. Sun, T. Feng and T. P. Russell, Langmuir, 2013, 29, 13407 CrossRef CAS PubMed.
- X. H. Song, Y. F. Yang, J. C. Liu and H. Y. Zhao, Langmuir, 2011, 27, 1186 CrossRef CAS PubMed.
- M. M. Gudarzi and F. Sharif, Soft Matter, 2011, 7, 3432 RSC.
- Y. Q. He, F. Wu, X. Y. Sun, R. Q. Li, Y. Q. Guo, C. B. Li, L. Zhang, F. B. Xing, W. Wang and J. P. Gao, ACS Appl. Mater. Interfaces, 2013, 5, 4843 CAS.
- S. C. Thickett and P. B. Zetterlund, J. Colloid Interface Sci., 2015, 442, 67 CrossRef CAS PubMed.
- K. Y. A. Lin, H. Yang, C. Petit and W. D. Lee, J. Colloid Interface Sci., 2015, 438, 296 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offerman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- X. Y. Gu, Y. Ning, Y. Yang and C. Y. Wang, RSC Adv., 2014, 4, 3211 RSC.
- A. J. Scarlett, W. L. Morgan and J. H. Hildebrand, J. Phys. Chem., 1926, 31, 1566 CrossRef.
- G. N. Yin, Z. Zheng, H. T. Wang, Q. G. Du and H. D. Zhang, J. Colloid Interface Sci., 2013, 394, 192 CrossRef CAS PubMed.
- M. Alanyalioglu, J. J. Segura, J. Oró-Solè and N. Casañ, Carbon, 2012, 50, 142 CrossRef CAS.
- Y. H. Xue, H. Chen, D. S. Yu, S. Y. Wang, M. Yardeni, Q. B. Dai, M. M. Guo, Y. Liu, F. Lu, J. Qu and L. M. Dai, Chem. Commun., 2011, 47, 11689 RSC.
- A. San-Miguel and S. H. Behrens, Langmuir, 2012, 28, 12038 CrossRef CAS PubMed.
- Y. Hu, X. Y. Gu, Y. Yang, J. Huang, M. Hu, W. K. Chen, Z. Tong and C. Y. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 17166 CAS.
- C. K. Chua and M. Pumera, Chem. Soc. Rev., 2014, 43, 291 RSC.
- Q. Wang, X. F. Guo, L. C. Cai, Y. Cao, L. Gan, S. Liu, Z. X. Wang, H. T. Zhang and L. D. Li, Chem. Sci., 2011, 2, 1860 RSC.
- G. X. Zhao, J. X. Li, L. Jiang, H. L. Dong, X. K. Wang and W. P. Hu, Chem. Sci., 2012, 3, 433 RSC.
- H. Zhang, X. F. Fan, X. Quan, S. Chen and H. T. Yu, Environ. Sci. Technol., 2011, 45, 5731 CrossRef CAS PubMed.
- Z. S. Wu, S. B. Yang, Y. Sun, K. Parvez, X. L. Feng and K. Müllen, J. Am. Chem. Soc., 2012, 134, 9082 CrossRef CAS PubMed.
- M. Losurdo, C. Yi, A. Suvorova, S. Rubanov, T. H. Kim, M. Giangregorio, W. Y. Jiao, I. Bergmair, G. Bruno and A. S. Brown, ACS Nano, 2014, 8, 3031 CrossRef CAS PubMed.
- L. L. C. Wong, S. Barg, A. Menner, P. D. V. Pereira, G. Eda, M. Chowalla, E. Saiz and A. Bismarck, Polymer, 2014, 55, 395 CrossRef CAS.
- S. W. Zou, H. Liu, Y. Yang, Z. J. Wei and C. Y. Wang, React. Funct. Polym., 2013, 73, 1231 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of nanoparticle-decorated GO sheets, Pickering emulsion and porous foam. See DOI: 10.1039/c5ra18397a |
| This journal is © The Royal Society of Chemistry 2015 |