Using maximum entropy approach for prediction of drop size distribution in a pilot plant multi-impeller extraction contactor
Abstract
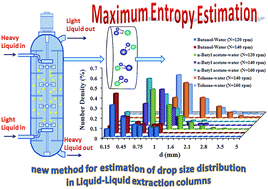
In this study, the maximum entropy principle is used to predict the drop size distributions in a multi-impeller column extractor. Three systems, including toluene–water, n-butyl acetate–water and n-butanol–water, were tested in this column. An experimental study of mass transfer conditions was performed in which acetone was transferred between the organic and aqueous phases. The drop size distribution and Sauter mean drop diameter were found to depend largely on the rotor speed and interfacial tension, but were only partially dependent on the phase velocities. Empirical correlations are proposed to describe Lagrange multipliers in the maximum entropy function in terms of the operating variables and physical properties of the systems. In addition, an empirical correlation is proposed for estimation of the Sauter mean drop diameter. Also, the combination of computational fluid dynamics (CFD) and droplet population balance modeling (PBM) has been carried out to predict the drop size distributions. Comparison shows good agreement between the present models and the experimental data. Experimental results show that the maximum entropy function satisfies the droplet size distributions for three systems in a multi-impeller column extractor. The acquired information would be useful in the design of liquid–liquid extraction columns.

 Please wait while we load your content...
Please wait while we load your content...