Degradation of soil-adsorbed DDT and its residues by NZVI addition
Swatantra Pratap Singh and
Purnendu Bose*
Environmental Engineering and Management Programme, Department of Civil Engineering, Indian Institute of Technology Kanpur, Kanpur – 208016, India. E-mail: pbose@iitk.ac.in; Tel: +91-512-2597403
First published on 28th October 2015
Abstract
Dichlorodiphenyltrichloroethane (DDT) is a highly persistent and toxic chlorinated pesticide. Market-grade DDT is a mixture of 4,4-DDT (85%), 2,4-DDT (15%) and trace amounts of 4,4-DDD, 2,4-DDD, 4,4-DDE and 4,4-DDMU. This mixture is commonly known as DDT and its residues, i.e., DDTr compounds. Due to their strongly hydrophobic nature, DDTr compounds are mostly partitioned into soil and sediments in the natural environment. Preliminary aqueous phase experiments showed that DDT and DDD were degraded by NZVI, with the degradation rates being 2,4-DDT > 4,4-DDT > 2,4-DDD > 4,4-DDD. NZVI addition to soil contaminated with DDTr compounds resulted in rapid reduction in soil-phase 4,4-DDT and 2,4-DDT concentrations and increase in soil-phase 4,4-DDD and 2,4-DDD concentrations, indicating conversion of 2,4-DDT to 2,4-DDD and 4,4-DDT to 4,4-DDD. Multiple addition of NZVI resulted in complete degradation of soil phase 4,4-DDT and 2,4-DDT and reduction in concentrations of 4,4-DDD and 2,4-DDD. Considering the extremely hydrophobic nature of DDTr compounds and their consequent unavailability in the aqueous phase, only direct soil-phase interaction between DDTr compounds and NZVI can explain these experimental observations. A mathematical model incorporating soil phase DDTr–NZVI interactions could explain and simulate the experimental data adequately. Mass balance on DDTr concentrations in soil indicated that ∼40 percent of the DDTr initially present in soil could be removed through the first NZVI addition. Further NZVI additions were successively less effective in removing DDTr from soil and after four successive additions of NZVI, ∼64% reduction in soil-phase DDTr concentration was achieved.
1. Introduction
Nano Zero Valent Iron (NZVI) particles have been extensively used for the reductive degradation/transformation of chlorinated organic compounds, e.g., chlorinated methanes, ethanes and ethenes, aromatic compounds, and pesticides1–4 and metals, i.e., Cr, As, U etc.5,6 The high surface area to volume ratio of NZVI promotes rapid electron transfer to contaminants resulting in degradation/transformation.7–10 In situ application of NZVI for remediation of contaminated subsurface sites has also been attempted in numerous cases.11–14 To prevent agglomeration of NZVI and increase its mobility in porous media, various surface modification techniques including coating of NZVI particles with polymers have been reported.15–17NZVI-contaminant interaction is reported to occur mainly in the aqueous phase and involves transport of the dissolved contaminant molecule to the NZVI particles suspended in aqueous phase for transfer of electrons.10,18,19 However, NZVI-contaminant interactions in porous media/soil-slurry systems have not been studied extensively and hence complete elucidation NZVI-contaminant interaction in such systems is not available.17,20,21 Nonetheless, the effectiveness of NZVI in porous media is thought to be governed by the mobility of NZVI particles and by the rate of desorption of pollutants from soil phase,21 i.e., availability of target contaminants in the aqueous phase for interaction with NZVI. However, recent studies report relatively rapid degradation of many strongly hydrophobic pollutants, i.e. DDT and γ-HCH in soil slurry systems through NZVI addition.22,23 Approximately 76% degradation of γ-HCH in a soil-slurry was reported in 3 hours.22 Similar observations with other strongly hydrophobic compounds, i.e., DDT, dinitrotolune, RDX etc. are also available.24,25 Since these strongly hydrophobic compounds are unlikely to desorb rapidly and hence would not be available in large concentrations in aqueous phase, rapid degradation of such compounds in soil-slurry systems cannot be explained solely by the aqueous phase interaction between NZVI and contaminants.
Dichlorodiphenyltrichloroethane (DDT) is a highly persistent chlorinated pesticide which was extensively used from 1950–1980 for control of agricultural pests and disease vectors.26–29 Market-grade DDT is a mixture of mainly 4,4-DDT (85%), 2,4-DDT (15%) along with trace amounts of 2,2-DDT, 4,4-DDD, 2,4-DDD and trace quantities of 4,4-DDE and 4,4-DDMU.23,30,31 This mixture is commonly known as DDT and its residues, viz., DDTr. Due to their strong hydrophobic nature, DDTr compounds are mostly partitioned to the soils and sediments in natural environments and hence dissolved concentrations of DDTr compounds in natural water bodies are very low.23,30–33 Due to its hazardous34–38 and persistent nature,37,39–44 DDT is banned in many countries,45,46 and it has also been declared as one of the ‘dirty dozen’ persistent organic pollutants (POPs) in the Stockholm Convention on POPs.46 However, DDT is still being used in developing countries like India, South Africa, etc., in a limited way, mainly for mosquito control in connection with the malaria eradication program.26,27,46–48 In many areas, historical and continued use of DDT has resulted in widespread DDTr contamination of agricultural soil, sediments and every level of the food chain.29,34,45 Natural attenuation of soil adsorbed DDTr compounds by physical–chemical processes and by biodegradation is very slow.28–31,34 A recent study on degradation of soil-adsorbed DDTr indicated that lack of bioavailability of DDTr compounds is the main reason for their slow biodegradation, even when the conditions are otherwise favourable.23
Recent studies have indicated that DDT can be degraded effectively through addition of zero-valent metallic particles. DDT was readily degraded by Fe/Ni bimetallic nano-particles to DDD and DDE in aqueous systems where the solubility of DDT was artificially enhanced through surfactant addition.49 Soil-adsorbed DDTr concentration was reduced by ∼40 percent in 28 hours through NZVI addition.23 El-Temesh et al.,20 applied NZVI suspensions to columns containing DDT contaminated soil and reported 45 percent reduction in DDT concentration. However, concentration of DDT degradation by-products, i.e., DDD and DDE, in the soil increased. In another related study,50 DDT degradation was reported to be less in aged DDT contaminated soil as compared to soil where DDT was recently added. DDT degradation with micron-size zero-valent iron particles was reported to be slower as compared with NZVI.51 Combination of solvent extraction and catalytic hydro-dechlorination (Pd/C) was used for the effective treatment of DDTr contaminated soil.52 In another study, ZVI was added as an amendment to enhance the biodegradation of soil-adsorbed DDTr compounds.53 In a system containing ZVI and oxygen 60–80% removal of DDT was achieved in 12 hours through a “Fenton-Like” process.54
Above studies indicate that degradation of soil-adsorbed DDT by NZVI addition is indeed possible, though the exact mechanism of such degradation needs to be elucidated, considering low solubility of DDT in water. The main objective of the present study was to understand and quantify NZVI-mediated degradation of DDT and consequent by-product formation in a soil-slurry system and elucidate the reaction and transport mechanisms involved in such interactions through mathematical modelling.
2. Materials and methods
2.1 Chemicals and glassware
The market grade DDT used for the experiments was procured from Hindustan Insecticides Limited, Mumbai, India. All pesticide standards (2,4 DDT, 4,4 DDT, 2,4 DDD and 4,4 DDD) and the internal standard 2,4,5,6-tetrachloro-m-xylene (>99% purity) were purchased from Sigma-Aldrich, India. The standards were used for quantification of DDT and its residues in soil and water by gas chromatographic analysis. All solvents used for sample preparation, i.e., n-hexane, acetonitrile and acetone (>99% purity, HPLC grade) were procured from Merck, India. All the other chemicals used for the sample preparation and extraction were purchased from Loba Chemicals, India.Borosilicate glass vials (ASTM type-I, Wheaton Science, Millville, NJ, USA) of 40 mL volume and equipped with screw caps and teflon faced re-sealable septa were used for various experiments. Disposable gas chromatograph (GC) auto sampler vials of 2 mL capacity and with 11 mm aluminium seals and PTFE rubber lined septa (Wheaton Science, USA) were used for sample storage before gas chromatographic (GC) analysis.
2.2 Soil and NZVI preparation
Soil with no prior exposure to pesticides was collected from a depth of 30–35 cm below the surface from the campus of the Indian Institute of Technology, Kanpur, India. Extraneous materials like twigs and grass were removed from the soil. The soil was then ground to remove lumps and the portion passing through a 1 mm sieve used further. The organic carbon content and pH of this soil were 0.21 ± 0.06 percent and 8.12–8.23 respectively. The organic carbon was measured by TOC analyzer (TOC–CPN, Shimadzu, Japan). Sand, silt and clay percentage in the soil were 32.4 ± 1.6, 63.8 ± 2.1 and 3.8 ± 0.4 respectively. Preliminary experiments showed that this soil contained no adsorbed DDTr compounds. Varying quantities of market grade DDTr was loaded on this soil using a procedure described in detail elsewhere.23 Briefly, the process involved thorough mixing of the soil with an emulsion of water and turpentine containing dissolved DDTr compounds. The soil slurry thus produced was dewatered by decantation and drying to obtain the DDT-loaded soil. Three soil samples, Soil-A, Soil-B and Soil-C containing 0.24, 0.18 and 0.12 μmol DDTr per g soil were prepared in this way. Percentage distribution for DDT and metabolites were as follows; 79.2 ± 2.3% 4,4-DDT, 11.2 ± 1.7% 2,4-DDT, 6.4 ± 0.8% 4,4-DDD, 0.8 ± 0.3% 2,4-DDD, 1.7 ± 0.4% 4,4-DDE and 0.57 ± 0.18% 4,4-DDMU.After DDTr loading, several 2 g portions of the soil samples were vortex mixed with 40 mL water and kept overnight. Subsequently, the supernatant was centrifuged and analyzed for DDTr compounds. Since, no DDTr was detected in the supernatant, it was concluded that the DDTr was strongly adsorbed on soil and DDTr desorption from the soil matrix, if any, was negligible in the short term. Several literature reports also indicate that desorption of DDTr from soil to aqueous phase is a very slow process.23,30,31
Preparation of NZVI was by a wet chemical process involving reduction of FeCl3 by sodium borohydride (NaBH4) as described elsewhere.33,55 Starch was used as a stabilizer to prevent agglomeration of NZVI particles. Characterization of NZVI particles was by Transmission Electron Microscopy (TEM) and BET surface area analyser and these results have been presented elsewhere.23 The NZVI particle size was found to vary between 11 nm to 40 nm and the average particle size was calculated to be 18.4 nm. Specific surface area of NZVI particles was determined to be 27.54 m2 g−1. An aliquot of the NZVI suspension was digested in nitric acid and the iron concentration in the digested solution was measured by atomic absorption spectroscopy to be ∼0.1 g L−1.
2.3 Aqueous phase DDTr–NZVI experiments
Concentrated stock solutions of various DDTr components, i.e., 4,4-DDT, 2,4-DDT, 4,4-DDD, and 2,4-DDD were prepared in acetone. NZVI suspension (0.1 g L−1) in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and acetone was taken in a 40 mL vial with no headspace. A small volume of a stock solution was added using a micro syringe such that the concentration of the compound in the vial was between 200–400 ppb. The vial was then capped tightly. All the above operations were carried out in a glove box and in a nitrogen atmosphere. Six vials were prepared for each compound, along with controls containing no NZVI. Two controls were kept aside for measurement of initial concentration. Other vials were put on a rotating shaker operating at 30 rpm such that the vial axis was horizontal at all times. Vials were removed, in duplicate along with one control, from the shaker at specified times for sampling and analysis. Ambient temperature was 31 ± 3 °C during these experiments. pH of solution was not controlled during these experiments, but was determined to be in the 8.0–8.5 range in all vials.
1 mixture of water and acetone was taken in a 40 mL vial with no headspace. A small volume of a stock solution was added using a micro syringe such that the concentration of the compound in the vial was between 200–400 ppb. The vial was then capped tightly. All the above operations were carried out in a glove box and in a nitrogen atmosphere. Six vials were prepared for each compound, along with controls containing no NZVI. Two controls were kept aside for measurement of initial concentration. Other vials were put on a rotating shaker operating at 30 rpm such that the vial axis was horizontal at all times. Vials were removed, in duplicate along with one control, from the shaker at specified times for sampling and analysis. Ambient temperature was 31 ± 3 °C during these experiments. pH of solution was not controlled during these experiments, but was determined to be in the 8.0–8.5 range in all vials.
2.4 NZVI-soil adsorbed DDTr experiments
The impact of NZVI addition on soil adsorbed DDTr concentration was investigated through experiments which involved both single and multiple additions of NZVI to the DDT-loaded soil. For the single NZVI addition experiment, 2 g (dry weight) of DDTr-loaded soil was taken in 40 mL vial. The remaining volume of vials was then filled with NZVI suspension (0.1 g L−1) such that no head space existed, and tightly capped. Control vials were prepared with 2 g (dry weight) of DDTr-loaded soil and Milli-Q water without any added NZVI. All the above processes were carried out in a glove box under nitrogen atmosphere. Two controls vials were set aside for measurement of initial concentrations. All other vials were put in a rotating shaker operating at 30 rpm such that the vial axis was horizontal at all times. Vials were removed from the shaker, in duplicate along with one control, every two hours for a period of 28 hours for sampling and analysis. Ambient temperature was 31 ± 3 °C during these experiments. The pH of solution was not controlled during these experiments, but was determined to be in the 7.5–8.5 range in all vials.In the multiple NZVI addition experiments, vials prepared as above were put on the rotating shaker for 48 hours, after which all vials were removed. The contents of two vials and one control were kept aside for sampling and analysis. The aqueous phase in the other vials was separated by centrifugation and removed. The remaining volume of these vials were refilled with either NZVI suspension (0.1 g L−1) or Milli-Q water (for control vials), capped tightly and put back on the mixer for a further 48 hour period. This procedure was repeated for 4 cycles with all three soils i.e. Soil-A, Soil-B and Soil-C.
2.5 DDTr extraction
For DDTr extraction from the aqueous phase, a 5 mL aliquot was added to hexane (water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane ratio was 1
hexane ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) followed by 15 minutes vortex mixing. This mixture was centrifuged at 5000 rpm for better phase separation. Finally, 2 mL of the hexane extract was stored in a sealed GC vial for further analysis. Percentage recovery for 4,4-DDT, 2,4-DDT, 4,4-DDD, 2,4-DDD were 95 ± 3, 96 ± 5, 93 ± 6 and 95 ± 4 respectively.
4) followed by 15 minutes vortex mixing. This mixture was centrifuged at 5000 rpm for better phase separation. Finally, 2 mL of the hexane extract was stored in a sealed GC vial for further analysis. Percentage recovery for 4,4-DDT, 2,4-DDT, 4,4-DDD, 2,4-DDD were 95 ± 3, 96 ± 5, 93 ± 6 and 95 ± 4 respectively.
In case of NZVI-soil adsorbed DDTr experiments, the solid and liquid phases were initially separated by centrifugation. DDTr concentration in the solid phase was measured using a modified version on the QuECheRS extraction procedure.56,57 The exact details and validation of the extraction procedure are given elsewhere.23 In summary, the procedure involved initial extraction of the soil-adsorbed DDTr in acetonitrile, followed by evaporation of the extract to dryness and reconstitution in n-hexane, and the extraction efficiencies were comparable to Soxhlet extraction procedure.23
2.6 Sample analysis
The DDTr concentration in all extracted samples was measured using a gas chromatograph (Model Clarus 500, Perkin-Elmer, USA) equipped with an electron capture detector and a capillary column (Elite-5) of size 30 m × 0.28 mm × 0.25 μm. Conditions for analysis were the identical to those described in Singh et al.23 All analysis was performed in split less mode and injection volume was 1 μL. Nitrogen gas of high purity was used as the carrier and makeup gas. Flow rate of makeup gas was about 30 mL min−1. The temperature of injector and the electron capture detector (ECD) were 250 °C and 375 °C respectively. The programme temperature for the oven was as follows; initial temperature 150 °C with 1 minute hold, ramp from 150 °C to 220 °C at 12 °C min−1, hold at 220 °C for 15 minutes, ramp from 220 °C to 300 °C at 15 °C min−1, hold at 300 °C for 2 minutes. Detection limit was 1 pg μL−1 for 4,4-DDT, 2,4-DDT, 4,4-DDD, 2,4-DDD. 2,4,5,6-Tetrachloro-m-xylene was used as internal standard. Samples were diluted as required before analysis. Results are sometimes reported in terms of the individual DDTr components and sometimes as the sum of all DDTr components. The soil-adsorbed DDTr concentrations were normalized using the dry weight of the corresponding soil samples.3. Results and discussion
3.1 Aqueous phase DDTr–NZVI interactions
In aqueous phase experiments, approximately 93% and 96% degradation for 4,4-DDT and 2,4-DDT respectively was observed in the experimental duration of 36 hours. The main degradation products formed were 4,4-DDD and 2,4-DDD respectively, however formation of degradation products was not equi-molar, indicating formation of unidentified by-products. In separate experiments, the extent of degradation of 4,4-DDD and 2,4-DDD was determined to be 65% and 77% respectively over an experimental duration of 168 hours. The degradation rate was pseudo-first order in all cases and the computed reaction rate constants (krd) are shown in Table 1. The degradation rate constants decreased in the following order, 2,4-DDT > 4,4-DDT > 2,4-DDD > 4,4-DDD. These aqueous phase experiments prove that NZVI can degrade 4,4-DDT and 2,4-DDT with the formation of 4,4-DDD and 2,4-DDD respectively as the main degradation products. Further, both 4,4-DDD and 2,4-DDD are also degraded by NZVI at a slower rate.| Compound | krd (aqueous phase degradation rates) h−1 | ki, μmol g−1 h−1 | ksi, μmol g−1 | kr h−1 |
|---|---|---|---|---|
| 4,4-DDT | 9.68 × 10−2 | 0.022 | 0.107 | 0.04 |
| 2,4-DDT | 1.16 × 10−1 | 0.023 | 0.129 | 0.04 |
| 4,4-DDD | 6.27 × 10−3 | 0.027 | 1.259 | 0.04 |
| 2,4-DDD | 8.64 × 10−3 | 0.025 | 0.567 | 0.04 |
3.2 NZVI-soil adsorbed DDTr interactions
3.3 Mechanism of DDTr–NZVI interaction in soil-slurry systems
The interaction between NZVI and contaminants is generally reported to occur through the transport of the dissolved contaminant molecule to the NZVI particle surface, where electron transfer results in the degradation/transformation of the contaminant molecule.58,59 In porous media or soil-slurry systems where contaminants can be adsorbed on soil, the extent of NZVI-contaminant interaction will depend on the availability of contaminant in the aqueous phase. For moderately hydrophobic contaminants, it is postulated that reduction in contaminant concentration in the aqueous phase through NZVI action leads to disruption of the adsorption–desorption equilibrium and the resulting desorption of contaminants from the soil phase results in continued NZVI-contaminant interaction.58,60Considering the above mechanism, the relatively rapid NZVI–DDTr interaction observed in the present study would imply relatively rapid desorption of DDTr components from soil, which seems unlikely due to the following reasons; first, octanol-water partition coefficients (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow) for 4,4-DDT and 2,4-DDT are 6.91 and 6.79 respectively.61 Consequently, these compounds are highly hydrophobic and have solubilities of only 25 μg L−1 and 85 μg L−1 in water respectively.61 Second, the rate of desorption of DDT from soil to aqueous phase has been reported to be very slow.23,30–32 Third, the aqueous phase DDT concentrations as measured during the present study were always below detection limits. Hence, it is unlikely that DDT desorption from the soil can occur at a rate consistent with the relatively rapid reduction in soil phase DDT concentrations observed in the present study.
Kow) for 4,4-DDT and 2,4-DDT are 6.91 and 6.79 respectively.61 Consequently, these compounds are highly hydrophobic and have solubilities of only 25 μg L−1 and 85 μg L−1 in water respectively.61 Second, the rate of desorption of DDT from soil to aqueous phase has been reported to be very slow.23,30–32 Third, the aqueous phase DDT concentrations as measured during the present study were always below detection limits. Hence, it is unlikely that DDT desorption from the soil can occur at a rate consistent with the relatively rapid reduction in soil phase DDT concentrations observed in the present study.
Degradation of soil adsorbed DDT as reported in this study can be explained by invoking a direct electron transfer mechanism between DDTr adsorbed on soil and NZVI particles. Literature reports indicate that due to their extreme small size, NZVI particles can be transported to the soil surface on which DDTr is adsorbed. In a relevant study,21 more than 70% of CMC stabilised NZVI was observed to be attached on soil in batch mixing condition. This is consistent with a scenario where a soil surface with a random distribution of adsorbed DDTr molecules is being randomly impacted by NZVI particles. DDTr degradation occurs when an NZVI particle impacts an adsorbed DDTr molecule.
At initial stages, soil phase concentration of DDTr is high and hence the rate of such interaction appears zero-order; at later stages, the rate of interaction becomes increasingly dependent on the residual soil phase DDTr concentration and thus approaches first order. Such a degradation regime can be represented by a rate expression of the type,
 | (1) |
 | (2) |
 | (3) |
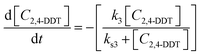 | (4) |
 | (5) |
In the above expressions, C4,4-DDT, C4,4-DDD, C2,4-DDT and C2,4-DDD are the soil phase concentrations (in μmol g−1) of 4,4-DDT, 4,4-DDD, 2,4-DDT and 2,4-DDD respectively, k1 and ks1 are the degradation rate constants of 4,4-DDT, k2 and ks2 are the degradation rate constants of 4,4-DDD, k3 and ks3 are the degradation rate constants of 2,4-DDT and k4 and ks4 are the degradation rate constants of 2,4-DDD. Further, f1 and f2 are the fractional conversions of 4,4-DDT to 4,4-DDD and 2,4-DDT to 2,4-DDD respectively. The initial conditions for solving the above equations are the initial concentrations (at t = 0) of the above compounds in soil.
Simulations were carried out using the above model (eqn (2)–(5)) in MATLAB R2014a (ode45) to explain the observed experimental data. Simulations (S1) of soil phase concentrations for 4,4-DDT, 4,4-DDD, 2,4-DDT and 2,4-DDD for Soil-A are shown in Fig. 1a and b. The fractional conversion values, i.e., f1 and f2, for 4,4-DDT and 2,4-DDT were taken as 0.75 and 0.65 respectively. The ki and ksi values were the fitting parameters and as obtained through the least square procedure. These values for various compounds are given in Table 1. Based on the comparison of the experimental data and S1 results it was concluded that while simulation results matched well with experimental data during the first few hours of the experiment, at later stages, the simulation results tended to over-predict the experimental results.
This over-prediction of the extent of degradation at later stages of the experiments probably indicates a loss in effectiveness of NZVI vis-a-vis its ability to degrade the target contaminants, a phenomenon not accounted in the simulations. Such loss of effectiveness of NZVI is to be expected in a complex system like soil slurry, NZVI particles being very reactive and amenable to transfer electrons to species other than the target compounds in the system.47,62,63 Transfer of electrons result in the conversion of the metallic iron on NZVI surface to iron oxide, which results in the passivation of the NZVI surface, i.e., loss of effectiveness of the NZVI surface to further transfer electrons. A passivation factor (Ru) was introduced in the model to account for this phenomenon, where,
| Ru = exp[−krt] | (6) |
The experimental data obtained through multiple addition experiments with Soil-A, Soil-B and Soil-C could also be effectively simulated using the modified model proposed above and the rate parameters in Table 1. In these experiments, NZVI was added to the soil sample on the 1st, 3rd, 5th and 7th day of experiment. Simulations corresponding to each addition were done separately, with the residual concentration of contaminants after 1st addition being taken as the initial conditions for the 2nd addition and so on. As shown in Fig. 2a–f the experimental data and model simulations match well.
3.4 Degradation of DDTr
Mass balance of DDTr compounds, i.e., sum of 4,4-DDT, 2,4-DDT, 4,4-DDD and 2,4-DDD concentrations present in Soil-A, Soil-B and Soil-C initially and after each NZVI addition was performed (Fig. 3). The results presented in Fig. 3 indicate that in all cases, nearly 40 percent of the DDTr concentration initially present on could be removed through the first NZVI addition. The percent removal increased to ∼50 percent, >55 percent and >60 percent respectively after the second, third and fourth NZVI addition. These results indicate that the first NZVI addition was most effective in removing DDTr for soil and the incremental increase in the percent of DDTr removal decreased with successive NZVI additions. Under the conditions of the present study, more than 4 additions of NZVI will not result in substantial increase in the removal of DDTr compounds from soil. Reduction of DDTr in soil may indicate mineralization or formation of unidentified lower metabolites of DDTr degradation.4. Conclusions
NZVI addition to DDTr compounds dissolved in acetone–water solution indicated that these compounds can be degraded by NZVI. NZVI addition to soil-adsorbed DDTr compounds resulted in relatively rapid degradation of 4,4-DDT and 2,4-DDT and increase in the concentration of 4,4-DDD and 2,4-DDD adsorbed on soil. Multiple addition of NZVI resulted in complete degradation 4,4-DDT and 2,4-DDT and reduction in concentration of 4,4-DDT and 2,4-DDT adsorbed on soil. Main conclusions of the study were as follows,• In experiments involving NZVI addition to DDTr dissolved in acetone–water solution, the degradation of all DDTr compounds was pseudo-first order. The degradation rates declined as follows, 2,4-DDT > 4,4-DDT > 2,4-DDD > 4,4-DDD. Degradation rates of the latter two compounds were approximately an order of magnitude lower than the first two compounds.
• Experiments involving single addition of NZVI to soil adsorbed DDTr compounds showed relatively rapid degradation of 2,4-DDT and 4,4-DDT and an increase in the concentrations of 2,4-DDD and 4,4-DDD. Formation of 2,4-DDD and 4,4-DDD is attributed to the reductive dechlorination of the corresponding parent compounds, i.e., 2,4-DDT and 4,4-DDT by NZVI.
• Experiments involving multiple addition of NZVI to soil adsorbed DDTr compounds indicated complete degradation of 4,4-DDT and 2,4-DDT after the second NZVI addition. 4,4-DDD and 2,4-DDD concentrations in soil declined with successive NZVI additions.
• Mass balance of DDTr concentrations in soil showed that, nearly 40 percent of the DDTr concentration initially present on could be removed through the first NZVI addition. Further NZVI additions were successively less effective in removing DDTr from soil.
• Considering the extremely hydrophobic nature of DDTr compounds, its low solubility in water and its slow desorption rate from soil, aqueous phase DDT–NZVI interactions cannot explain the relatively rapid rate of DDT degradation observed in this study. Degradation of soil adsorbed DDT was explained by invoking a direct electron transfer mechanism between DDTr adsorbed on soil and NZVI particles.
• A model formulated incorporating soil-phase interaction between DDTr compounds and NZVI and a passivation factor to account for the loss of effectiveness of NZVI surface with time vis-à-vis contaminant degradation could adequately explain the DDTr degradation data obtained during this study.
Finally, this study indicates that NZVI addition may be effective in reducing the soil adsorbed concentration of strongly hydrophobic compounds such as DDTr from soil and the mechanism of such interaction involves direct interaction of NZVI particles with soil-adsorbed contaminants.
References
- W.-F. Chen, L. Pan, L.-F. Chen, Q. Wang and C.-C. Yan, RSC Adv., 2014, 4, 46689–46696 RSC.
- H. Song and E. R. Carraway, Environ. Sci. Technol., 2005, 39, 6237–6245 CrossRef CAS PubMed.
- Y. Liu, S. A. Majetich, R. D. Tilton, D. S. Sholl and G. V. Lowry, Environ. Sci. Technol., 2005, 39, 1338–1345 CrossRef CAS PubMed.
- G. Naja, A. Halasz, S. Thiboutot, G. Ampleman and J. Hawari, Environ. Sci. Technol., 2008, 42, 4364–4370 CrossRef CAS PubMed.
- H. K. Boparai, M. Joseph and D. M. O'Carroll, J. Hazard. Mater., 2011, 186, 458–465 CrossRef CAS PubMed.
- Y. Xu and D. Zhao, Water Res., 2007, 41, 2101–2108 CrossRef CAS PubMed.
- B. I. Kharisov, H. V. Rasika Dias, O. V. Kharissova, V. Manuel Jimenez-Perez, B. Olvera Perez and B. Munoz Flores, RSC Adv., 2012, 2, 9325–9358 RSC.
- E. J. Petersen, R. A. Pinto, X. Shi and Q. Huang, J. Hazard. Mater., 2012, 243, 73–79 CrossRef CAS PubMed.
- R. Cheng, J.-L. Wang and W.-X. Zhang, J. Hazard. Mater., 2007, 144, 334–339 CrossRef CAS PubMed.
- G. V. Lowry and K. M. Johnson, Environ. Sci. Technol., 2004, 38, 5208–5216 CrossRef CAS PubMed.
- N. Mueller, J. Braun, J. Bruns, M. Černík, P. Rissing, D. Rickerby and B. Nowack, Environ. Sci. Pollut. Res., 2012, 19, 550–558 CrossRef CAS PubMed.
- F. Fagerlund, T. Illangasekare, T. Phenrat, H.-J. Kim and G. Lowry, J. Contam. Hydrol., 2012, 131, 9–28 CrossRef CAS PubMed.
- T. Phenrat, F. Fagerlund, T. Illangasekare, G. V. Lowry and R. D. Tilton, Environ. Sci. Technol., 2011, 45, 6102–6109 CrossRef CAS PubMed.
- A. Taghavy, J. Costanza, K. D. Pennell and L. M. Abriola, J. Contam. Hydrol., 2010, 118, 128–142 CrossRef CAS PubMed.
- H.-Y. Shu, M.-C. Chang, C.-C. Chen and P.-E. Chen, J. Hazard. Mater., 2010, 184, 499–505 CrossRef CAS PubMed.
- Y.-H. Lin, H.-H. Tseng, M.-Y. Wey and M.-D. Lin, Sci. Total Environ., 2010, 408, 2260–2267 CrossRef CAS PubMed.
- K. Darko-Kagya, A. P. Khodadoust and K. R. Reddy, J. Hazard. Mater., 2010, 182, 177–183 CrossRef CAS PubMed.
- F. He and D. Zhao, Environ. Sci. Technol., 2005, 39, 3314–3320 CrossRef CAS PubMed.
- J. T. Nurmi, P. G. Tratnyek, V. Sarathy, D. R. Baer, J. E. Amonette, K. Pecher, C. Wang, J. C. Linehan, D. W. Matson, R. L. Penn and M. D. Driessen, Environ. Sci. Technol., 2004, 39, 1221–1230 CrossRef.
- Y. El-Temsah, D. Oughton and E. Joner, Plant Soil, 2013, 368, 189–200 CrossRef CAS.
- M. Zhang, F. He, D. Zhao and X. Hao, Water Res., 2011, 45, 2401–2414 CrossRef CAS PubMed.
- R. Singh, V. Misra, M. K. R. Mudiam, L. K. S. Chauhan and R. P. Singh, J. Hazard. Mater., 2012, 237–238, 355–364 CrossRef CAS PubMed.
- S. P. Singh, P. Bose, S. Guha, S. K. Gurjar and S. Bhalekar, Chemosphere, 2013, 92, 811–820 CrossRef CAS PubMed.
- G. Naja, R. Apiratikul, P. Pavasant, B. Volesky and J. Hawari, Environ. Pollut., 2009, 157, 2405–2412 CrossRef CAS PubMed.
- K. R. Reddy, K. Darko-Kagya and C. Cameselle, Sep. Purif. Technol., 2011, 79, 230–237 CrossRef CAS.
- L. J. Nadeau, G. S. Sayler and J. C. Spain, Arch. Microbiol., 1998, 171, 44–49 CrossRef CAS PubMed.
- T. Gyalpo, L. Fritsche, H. Bouwman, R. Bornman, M. Scheringer and K. Hungerbühler, Environ. Pollut., 2012, 169, 235–241 CrossRef CAS PubMed.
- H. L. Boul, N. Z. J. Agric. Res., 1995, 38, 257–277 CrossRef CAS.
- A. S. Purnomo, T. Mori, I. Kamei and R. Kondo, Int. Biodeterior. Biodegrad., 2011, 65, 921–930 CrossRef CAS.
- S. M. A. D. Zayed, I. Y. Mostafa and A. E. El-Arab, J. Environ. Sci. Health, Part B, 1994, 29, 47–56 CrossRef.
- S. E. Hale, J. E. Tomaszewski, R. G. Luthy and D. Werner, Water Res., 2009, 43, 4336–4346 CrossRef CAS PubMed.
- A. Ghauch, J. Rima, C. Amine and M. Martin-Bouyer, Chemosphere, 1999, 39, 1309–1315 CrossRef CAS PubMed.
- J. T. Nurmi, P. G. Tratnyek, V. Sarathy, D. R. Baer, J. E. Amonette, K. Pecher, C. Wang, J. C. Linehan, D. W. Matson and R. L. Penn, Environ. Sci. Technol., 2005, 39, 1221–1230 CrossRef CAS PubMed.
- M. López-Cervantes, L. Torres-Sánchez, A. Tobías and L. López-Carrillo, Environ. Health Perspect., 2004, 112, 207–214 CrossRef.
- Y.-R. Wang, M. Zhang, Q. Wang, D.-Y. Yang, C.-L. Li, J. Liu, J.-G. Li, H. Li and X.-Y. Yang, Sci. Total Environ., 2008, 396, 34–41 CrossRef CAS PubMed.
- T. L. Johnson, M. M. Scherer and P. G. Tratnyek, Environ. Sci. Technol., 1996, 30, 2634–2640 CrossRef CAS.
- A. Nair, T. Samuel and M. K. K. Pillai, Pestic. Sci., 1992, 34, 333–340 CrossRef CAS.
- Y.-H. Kim and E. R. Carraway, Environ. Sci. Technol., 2000, 34, 2014–2017 CrossRef CAS.
- G. M. Woodwell, P. P. Craig and H. A. Johnson, Science, 1971, 174, 1101–1107 CrossRef CAS PubMed.
- I. Scheunert, D. Vockel, J. Schmitzer and F. Korte, Chemosphere, 1987, 16, 1031–1041 CrossRef CAS.
- M. M. Andréa, R. Y. Tomita, L. C. Luchini and M. R. Musumeci, J. Environ. Sci. Health, Part B, 1994, 29, 133–139 CrossRef.
- G. Wedemeyer, Appl. Microbiol., 1967, 15, 569–574 CAS.
- M. S. Sharom, J. R. W. Miles, C. R. Harris and F. L. McEwen, Water Res., 1980, 14, 1095–1100 CrossRef CAS.
- P. G. Tratnyek, A. Salter-Blanc, J. Nurmi, J. E. Amonette, J. Liu, C. M. Wang, A. Dohnalkova and D. R. Baer, Reactivity of zerovalent metals in aquatic media: Effects of organic surface coatings, Pacific Northwest National Laboratory (PNNL), Environmental Molecular Sciences Laboratory (EMSL), Richland, WA (US), 2011 Search PubMed.
- UNEP-Chemicals, Journal, 2004.
- WHO, Journal, 2011.
- J. Gotpagar, E. Grulke, T. Tsang and D. Bhattacharyya, Environ. Prog., 1997, 16, 137–143 CrossRef CAS.
- G. R. Eykholt and D. T. Davenport, Environ. Sci. Technol., 1998, 32, 1482–1487 CrossRef CAS.
- T. Hua, L. Jinjun, M. Zhen, L. Landong and H. Zhengping, Sep. Purif. Technol., 2009, 66, 84–89 CrossRef.
- Y. S. El-Temsah and E. J. Joner, Chemosphere, 2013, 92, 131–137 CrossRef CAS PubMed.
- S.-C. Yang, M. Lei, T.-B. Chen, X.-Y. Li, Q. Liang and C. Ma, Chemosphere, 2010, 79, 727–732 CrossRef CAS PubMed.
- X. Ma, Y. Luan, S. Liu, Y. Liu and C. Xia, RSC Adv., 2015, 5, 42597–42602 RSC.
- X. Cong, N. Xue, S. Wang, K. Li and F. Li, Sci. Total Environ., 2010, 408, 3418–3423 CrossRef CAS PubMed.
- M. Cao, L. Wang, L. Wang, J. Chen and X. Lu, Chemosphere, 2013, 90, 2303–2308 CrossRef CAS PubMed.
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940–13941 CrossRef CAS PubMed.
- M. Anastassiades, K. Mastovska and S. J. Lehotay, J. Chromatogr., A, 2003, 1015, 163–184 CrossRef CAS PubMed.
- M. Anastassiades, S. J. Lehotay, D. Stajnbaher and F. J. Schenck, J. AOAC Int., 2003, 86, 412–431 CAS.
- L. J. Matheson and P. G. Tratnyek, Environ. Sci. Technol., 1994, 28, 2045–2053 CrossRef CAS PubMed.
- R. W. Gillham and S. F. O'Hannesin, Ground Water, 1994, 32, 958–967 CrossRef CAS.
- E. J. Weber, Environ. Sci. Technol., 1996, 30, 716–719 CrossRef CAS.
- ATSDR, Journal, 2012.
- B. Deng, D. R. Burris and T. J. Campbell, Environ. Sci. Technol., 1999, 33, 2651–2656 CrossRef CAS.
- W. A. Arnold and A. L. Roberts, Environ. Sci. Technol., 2000, 34, 1794–1805 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |



