Preparation and enhanced photoelectrochemical performance of a p–n heterojunction CuFe2O4/WO3 nanocomposite film†
Abstract
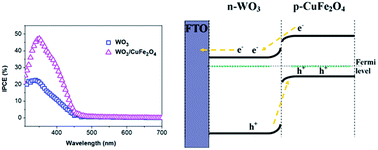
In this work, a p–n heterojunction film consisting of n-type WO3 and p-type CuFe2O4 was synthesized via two steps. The n-type WO3 film was deposited on the FTO substrate by a doctor-blade method and then modified with p-type CuFe2O4 nanoparticles by a deposition-annealing method. The composite film was characterized by X-ray powder diffraction, scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy and UV-vis diffuse reflectance spectroscopy, showing that the CuFe2O4 nanoparticles were deposited on the surface of WO3 film. Meanwhile, photoelectrochemical measurements were used to investigate the photoelectrochemical properties. A photocurrent of 0.75 mA cm−2 at 0.6 V (vs. Ag/AgCl) was achieved with CuFe2O4/WO3, resulting in a 2.68 fold increase compared to pristine WO3. The presence of a p–n heterojunction facilitates the separation of photoinduced electrons and holes, leading to more efficient charge transfer, resulting in a significant improvement in PEC performance.

 Please wait while we load your content...
Please wait while we load your content...