3-Hydroxypropionaldehyde (3-HPA) quantification by HPLC using a synthetic acrolein-free 3-hydroxypropionaldehyde system as analytical standard†
Abstract
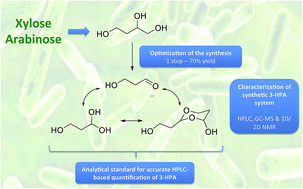
HPLC-based quantification of 3-HPA using a synthetic acrolein-free 3-HPA standard obtained from commercially available 1,2,4-butanetriol through a straightforward and easy synthetic process has advantages over previous colorimetric methods of easier and safer implementation, and greater specificity. This HPLC method is very simple to implement in a lab, does not need any extra handling of the sample to be analyzed, and is suitable even in the presence of other aldehydes and 3-HPA derivatives, provided that the latter do not have similar retention times.

 Please wait while we load your content...
Please wait while we load your content...