A study on the trastuzumab conjugation at tyrosine using diazonium salts
Nils Griebenow*a,
Simone Grevenb,
Mario Lobella,
Alicia M. Dilmaçac and
Stefan Bräse*cd
aBayer Pharma AG, Global Drug Discovery, Medicinal Chemistry Wuppertal, Aprather Weg 18a, 42113 Wuppertal, Germany
bBayer Pharma AG, Global Drug Discovery, Research Analytics, Aprather Weg 18a, 42113 Wuppertal, Germany
cKarlsruher Institut für Technologie (KIT), Institute of Organic Chemistry, Fritz-Haber-Weg 6, Campus Süd, D-76131 Karlsruhe, Germany. E-mail: stefan.braese@kit.edu; Fax: +49 (0) 721 608 48581; Tel: +49 (0) 721 608 42902
dKarlsruher Institut für Technologie (KIT), Institute of Toxicology and Genetics, Hermann-von-Helmholtz-Platz 1, D-76344 Eggenstein-Leopoldshafen, Germany
First published on 24th November 2015
Abstract
Herein, we report on the conjugation of trastuzumab with 2,5-difluorobenzene diazonium tetrafluoroborate. According to the amount of diazonium salt used, an average loading of 1.0 to 3.8 azo compounds per antibody was found. Tryptic digestion and subsequent MS–MS analyses allowed the identification of the main conjugation sites. Furthermore, additional conjugation reactions of trastuzumab with 4-formylbenzene diazonium hexafluorophosphate (FBDP) allows us to make a short study on the influence of the different reaction parameters on the outcome of the conjugation reaction, both in terms of antibody loading and of conjugation sites. In all cases, antibody conjugation was achieved with full selectivity towards tyrosine residues.
Introduction
In recent years, the synthesis of peptide- and protein conjugates as therapeutics, as diagnostics and as tools to elucidate biological mechanisms has become increasingly important.2 In particular, antibody drug conjugates (ADCs), which are especially designed to combine the specificity of an antibody for drug delivery with the high potency of a cytotoxic drug to which it has been covalently linked to, represent an ever growing area of research nowadays. Such a drug targeting therapy allows for sensitive discrimination between healthy and diseased tissue.2,3 The recent FDA approval of two drugs of this kind, ado-trastuzumab emtansine (Kadcyla®) and brentuximab vedotin (Adcetris®), proves the success of such a technology and justifies the ever growing interest in the design of new ADCs.Nevertheless, the synthesis of such conjugates can be challenging, as it requires a chemo selective methodology that should modify the targeted conjugation sites without altering neither the integrity of the antibody, nor the toxicity of the drug. To date, most of the reported ADC syntheses are performed by reacting antibodies at either their lysine or at their cysteine residues, as each of these amino acids can be easily modified in a selective manner. However, some drawbacks have been encountered in cases, among which statistical drug-to-antibody ratios (DAR), heterogeneity and unpredictable pharmacokinetic properties of the resulting conjugates.4 The difficulties encountered in these well-explored conjugation techniques justify the scientific urge to describe new bio conjugation methodologies for the conception of new ADCs.
While tyrosine modification has long been a major tool in the structural studies of proteins,5–12 its use in conjugation reactions has been less explored. Nonetheless, tyrosine presents interesting features for conjugation purposes. Firstly, its lower occurrence on the surface of proteins when compared to lysine let one expect a higher homogeneity in conjugation.10 Furthermore, the little, if none effect on the overall charge of the biomolecules conjugated at tyrosines is believed to be another positive asset of this amino acid as conjugation site.10 Numerous reactions have been recently explored for the modification of small peptides and proteins at their tyrosine residues, among which the alkylation of tyrosine phenolate using π-allyl complexes,11 one-pot iodination/Suzuki–Miyaura coupling reactions,12 a DMAP catalyzed acyl transfer reaction13 and various addition reactions onto imines.14–18 Unfortunately however, side reactions involving tryptophan,16 lysine19 or cysteine15,18 residues were reported in several of these cases. Although some of these side reactions appeared to be substrate dependent16 or could be overcome upon adaptation of the reaction conditions,15,18,19 to date, the chemo selectivity of most of these methodologies seems uncertain. More recently however, Barbas and co-workers20,21 described an elegant tyrosine conjugation methodology using the cyclic diazodicarboxamide 4-phenyl-3H-1,2,4-triazole-3,5(4H)-dione (PTAD) and analogues. Although tryptophan and lysine appeared to be also reactive towards this type of reagent, full selectivity towards tyrosine was observed in competitive conditions.20 Based on these promising results, Bauer et al.22 designed a bifunctional linker bearing a cyclic diazo-carboxamide moiety, which was successfully used in the synthesis of DNA–protein conjugates. Similarly, Hu et al.23 adapted this methodology to the glycosylation of diphtheria toxin CRM197, therefore describing a selective and reproducible glycoconjugate vaccine synthesis.
Yet, if tyrosine modification was so widely explored for the ligation of various peptides and proteins, to the best of our knowledge, only very rare studies have explored its use in the synthesis of ADCs. On one hand, Barbas and coworkers described the use of their aforementioned PTADs conjugation methodology for the synthesis of trastuzumab–RGD conjugates,20 and later for the conjugation of trastuzumab to the CCD5 inhibitor aplaviroc.21 On the other hand, they reported the synthesis of other trastuzumab conjugates using diazonium salts.1,24 Diazonium reagents are indeed known to react with aromatic compounds in a selective and efficient manner and stands as a perfect tool for tyrosine conjugation. When applied to peptide and protein conjugation, these reagents proved to be highly selective towards tyrosines, the possible side reactions with other amino acids (histidine, tryptophan cysteine and lysine) remaining minor at an appropriate pH.19,24,25 Although largely used within the last century for the affinity labeling of proteins and antibodies,5,6,26–28 diazonium salts were long set aside, as considered too unstable and difficult to handle to be used as an efficient bio conjugation tool. However, the successful work of Francis and coworkers25,29 to effectively modify viral capsids at tyrosine residues seem to have revived the interest in the use of this chemical method on antibodies. Since then, Barbas and coworkers proposed the use of stable tetrafluoroborate and hexafluorophosphate diazonium salts as the key to overcome the stability issues that limited the use of this technology, and, therefore, successfully applied it to the biotinylation of trastuzumab and to the synthesis of various antibodies–aplaviroc conjugates. It is interesting to see how this long known, yet avoided methodology allowed this team to conjugate different antibodies selectively at tyrosine residues, with antibody loadings1,24 reported to be lower than the one generally observed in lysine and cysteine conjugation reactions. Considering these promising results, we decided to further explore the use of diazonium reagents in the synthesis of antibody conjugates and report here a short study on the conjugation of trastuzumab with a new diazonium salt 3. We have selected diazonium salt 3, because it was found to be stable and could be stored for several months at −18 °C in our hands. Investigation of the conjugation of 3 brings additional information to the studies previously performed by Barbas and coworkers24 by revealing the possible influence of the nature and amount of the diazonium salt on the amount and location of modified tyrosine residues.
Results and discussion
Synthesis of diazonium salt 3
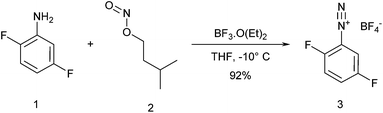
The use of diazonium salts in bio conjugation reactions has been long limited by their low stability, which makes them difficult to handle and generally requires their synthesis in situ prior to conjugation.24 However, tetrafluoroborate or hexafluorophosphate counter ions yield more stable compounds that can be easily stored and handled.24 Their use is therefore a now validated means to overcome the general stability issues of diazonium salts and has already been successfully used in syntheses of bio conjugates.1,24 Taking this into account, 2,5-difluorobenzene diazonium tetrafluoroborate 3 was chosen as a model reagent for the desired tyrosine conjugation. Given the fact that electron withdrawing groups on the aromatic ring can increase the reactivity of diazonium salts and are essential for efficient conjugation, this diazonium reagent was purposely chosen with two fluorines on its aromatic ring. While tyrosine conjugation had already been performed using various diazonium salts bearing various substituents (p-nitro, p-formyl, etc.), to the best of our knowledge, this is the first example of tyrosine conjugation using a fluorinated diazonium reagent. Diazonium salt 3 was thus easily synthetized by reacting 2,5-difluoroaniline with isoamylnitrit in the presence of boron trifluoride etherate (Scheme 1). Precipitation from ethyl acetate afforded diazonium salt 3 in 92% yield.Trastuzumab conjugates
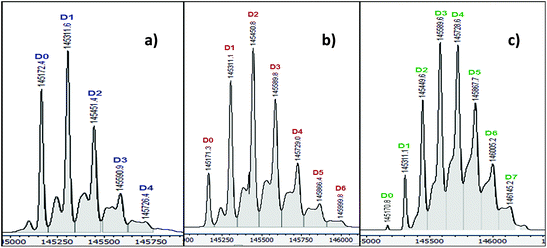
Trastuzumab (Herceptin®) is a humanized monoclonal antibody commonly used in ADC syntheses. This antibody has also been a template for most of the antibody conjugation reactions at tyrosines.1,20,21,24 For comparison purposes, trastuzumab was thus chosen here again as a model antibody for the study of antibody conjugation using diazonium salt 3.In our case, the conjugation reactions were performed in buffered solutions at physiological pH. An excess of diazonium salt 3 (3 to 10 eq.) was added to a 0.1 mM solution of trastuzumab in PBS buffer and allowed to react over 140 min at room temperature. After a prior purification step over Sephadex (G-25M) followed by ultrafiltration, characterization of the resulting conjugates by MS analyses revealed that the average amount of azo compound per antibody molecule was dependent on the amount of diazonium salts used (Table 1): an average of 1.4 modifications/antibody molecule was obtained when 3 equivalents of diazonium salt were used, while the average loading was 2.4 and 3.8 with 6 and 10 equivalents of this reagent, respectively. More specifically, the mass distribution obtained after each experiment showed the presence of antibodies bearing 0 to 4 azo compounds in each case (D0, D1, D2 and D3 in Fig. 1). Minor amounts of conjugates bearing 5 and 6 azo compounds (D5 and D6 in Fig. 1) were found with the use of 6 equivalents of diazonium 3, while up to 10 equivalents of the same reagent were required to observe a maximum load of 7 (Table 1 and Fig. 1). These results show the dependency of the antibody loading on the amount of diazonium salt used. Very interestingly, relative good homogeneity was achieved by limiting the amount of diazonium salt to three equivalents.
| Trastuzumab | Diazonium 3 | Distribution of azo compound per antibody | Average loading of the antibody | Main modification sites |
|---|---|---|---|---|
| 1 eq. | 3 eq. | 0 to 4 | 1.4 | Y33 |
| 1 eq. | 6 eq. | 0 to 6 | 2.4 | Y33, Y56 |
| 1 eq. | 10 eq. | 0 to 7 | 3.8 | Y33, Y56, Y79 |
Tryptic digestion and subsequent MS–MS analyses allowed the identification of the main conjugation sites. Modification at Y33 was observed in each case, independently of the amount of diazonium salt used. Y56 (Kabat Numbering)† was also modified in reactions using 6 or more equivalents of 3, while up to 10 equivalents of the reagent were required to observe conjugation at Y79. In this last case, some minor modifications could also be noticed on Y100A and Y436. These observations allowed us to conclude that conjugation of diazonium salt 3 with trastuzumab occurred preferentially at Y33, and only then at Y56 and Y79. Besides Y436, the conjugated tyrosine residues are all located on the heavy chain in the Fv region and, as expected, are all surface accessible (Fig. 2A). As shown in Fig. 2B, they also belong to the binding site of the antibody and are likely to be involved in Her2 binding. The influence of this conjugation in the Her2 binding of trastuzumab is currently being investigated in our laboratory and should be reported in due course. Noteworthy, no modification was ever observed at Y319 (Fc domain) in our case, while this residue was reported as being the main conjugation site in the conjugation of 4-formylbenzene diazonium hexafluorophosphate (FBDP) with many antibodies, including trastuzumab.1 These combined results highlight the dependency of the tyrosine conjugation location on the structure of the diazonium salt used.
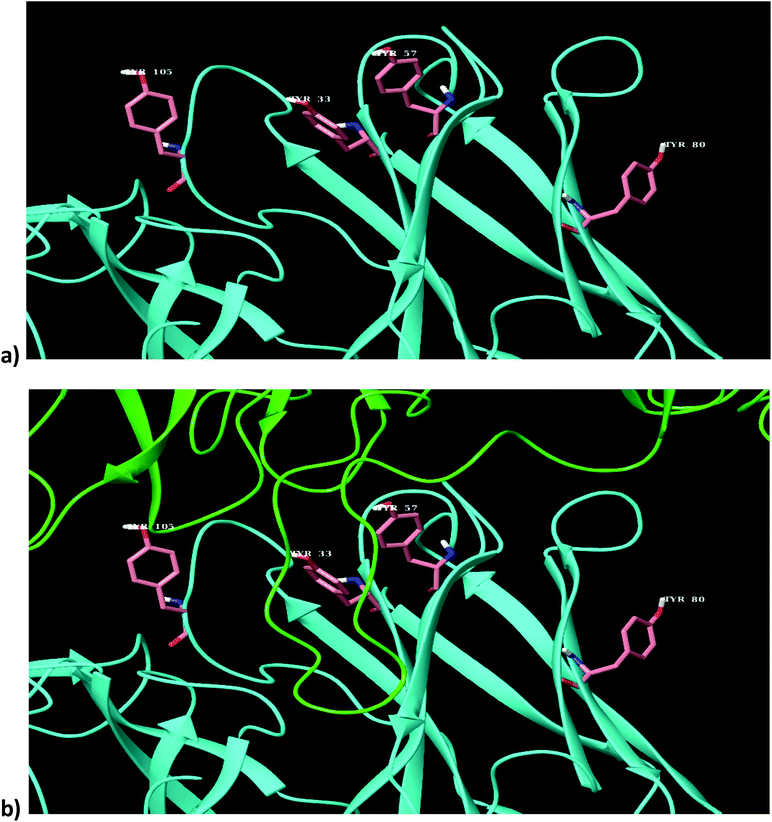 | ||
| Fig. 2 Representation of the tyrosine residues being modified in the reaction of trastuzumab with diazonium salt 3 and their involvement in Her2 binding. (A): Modified tyrosines 33 (TYR 33), 56 (TYR 57), 79 (TYR 80) and 100A (TYR 103) are freely accessible on the surface of the Fab domain (trastuzumab Fab domain X-ray crystal structure [4hjg.pdb] is shown as a light blue ribbon diagram with the four tyrosines shown in addition as stick models). (B): Modified tyrosines 33 (TYR 33), 56 (TYR 57), and 100A (TYR 103) of trastuzumab are most likely involved in Her2 binding (the X-ray crystal structure of pertuzumab with bound Her2 [1s78.pdb] has been aligned [based on matching secondary structure elements] and overlaid with trastuzumab [4hjg.pdb], shown are trastuzumab [blue ribbon] and Her2 [green ribbon], for clarity of view the overlaid pertuzumab is not shown in the picture). | ||
In order to further explore the difference of reactivity of these two diazonium salts, the tyrosine conjugation reaction of trastuzumab was repeated with 4-formylbenzene diazonium hexafluorophosphate at pH 7.1 and allowed to react with different length of time. The outcomes of these conjugation reactions are compared to the aforementioned results and to the literature data in Table 2.
| Entry | Trastuzumab | Diazonium | Time | pH | Distribution of azo compound | Average loading of the antibody |
|---|---|---|---|---|---|---|
| 1 | 1 eq. | FBDP (10 eq.) | 30 min | 8 | 0 to 3 | 1 |
| 2 | 1 eq. | FBDP (10 eq.) | 30 min | 7.1 | 0 to 3 | 0.5 |
| 3 | 1 eq. | FBDP (10 eq.) | 140 min | 7.1 | 0 to 5 | 1.9 |
| 4 | 1 eq. | Diazonium 3 (10 eq.) | 140 min | 7.1 | 0 to 7 | 3.8 |
Barbas et al.24 already reported the effect of the pH on the outcome of tyrosine conjugation reactions. Their experiments showed that the lower the pH, the lower the average number of modifications. Our results are thus in accordance with this study, and so is the average amount of modifications per antibody molecule (0.5) observed after 30 minutes of reaction with FBDP with the amount expected from their plot (number of modifications/proteins vs. pH).24 However, an approx. 5 times longer reaction time (140 minutes) (Table 2, entry 3) yielded an average loading of 1.9, with a higher heterogeneity (antibody species with up to 5 azo compounds were identified). This is a higher load than what would be expected from these literature data.24 Still, this value is lower than what was observed with diazonium salt 3, which tends to show the higher reactivity of the latter. As the tetrafluoroborate salt of formylbenzene diazonium was already reported to be less reactive than FBDP, this difference of reactivity between FBDP and diazonium salt 3 is probably independent of the counter ion of the respective salts, but rather a function of the substitution of their aromatic ring. This can probably be attributed to the higher electron withdrawing character of the two fluorines when compared to the formyl group in FBDP, which increase the reactivity of the reagent and, consequently, the distribution in loading.
Experimental
Material and methods
2,5-Difluorobenzene diazonium tetrafluoroborate 3. The reaction was run under an argon atmosphere. 2,4-Difluoroaniline was dissolved in 170 mL of THF (anhydrous) and added slowly to BF3·OEt2 at 0 °C. Isoamylnitrite was then dissolved in 30 mL THF (anhydrous) and added drop wise over 30 minutes at 0 °C, and the mixture was stirred overnight at the same temperature. It was then diluted with some diethylether. The resulting precipitate was filtrated, rinsed with a small amount of cold diethyl ether and dried under high vacuum (14.66 g, 92%).
General procedure for the diazonium salts modification of trastuzumab. The diazonium salt 3 (3, 6 or 10 eq.) was firstly dissolved in 2.130 mL of PBS buffer (pH 7.1) and then added to 370 μL of a trastuzumab solution in PBS buffer (13.5 mg mL−1). The mixture was stirred at rt for 140 minutes. The reaction mixture was then purified over a Sephadex column G25, which was equilibrated with PBS buffer. The resulting 3.5 mL of solution was centrifuged (5 min, 10 °C, 4000 tr min−1). The solution was then disposed into an Amicon Ultracel 30K centrifugal filter and concentrated over centrifugation (60 min, 10 °C, 4000 tr min−1). The resulting solution (volume < 250 μL) was diluted in PBS buffer to a volume of 2.5 mL. The number of modifications per antibody was determined by MS analyses and their main location after tryptic digestion and MS/MS based identification of the conjugated peptides.
Conclusions
Herein, we reported the tyrosine conjugation of trastuzumab using a new aryl diazonium salt: 2,5-difluorobenzene diazonium tetrafluoroborate. This study highlights the effect of the substitution of the aryl ring on both the number of modifications on the antibody and their locations. While the effects of the pH and of the reaction time on the conjugation outcome were reported previously and confirmed here again, to the best of our knowledge, an influence of the nature of the diazonium reagent was never mentioned until now. It is worth noticing that this conjugation methodology remained highly selective towards tyrosine, as the modification of other amino acids was never observed. A judicious choice of the reaction conditions also allows a fine tuning of the load distribution, in order to reach relatively good homogeneity. Thus, these results bring new insight to the factors influencing the tyrosine conjugation of antibodies, but also show the necessity of further investigations for a better understanding of this yet promising and site selective conjugation technique.Notes and references
- J. Gavrilyuk, H. Ban, H. Uehara, S. J. Sirk, K. Saye-Francisco, A. Cuevas, E. Zablowsky, A. Oza, M. S. Seaman, D. R. Burton and C. F. Barbas 3rd, J. Virol., 2013, 87, 4985–4993 CrossRef CAS PubMed.
- J. A. Flygare, T. H. Pillow and P. Aristoff, Chem. Biol. Drug Des., 2013, 81, 113–121 CAS.
- S. Panowksi, S. Bhakta, H. Raab, P. Polakis and J. R. Junutula, mAbs, 2014, 6, 34–45 CrossRef PubMed.
- K. J. Hamblett, P. D. Senter, D. F. Chace, M. M. Sun, J. Lenox, C. G. Cerveny, K. M. Kissler, S. X. Bernhardt, A. K. Kopcha, R. F. Zabinski, D. L. Meyer and J. A. Francisco, Clin. Cancer Res., 2004, 10, 7063–7070 CrossRef CAS PubMed.
- F. Franěk, Eur. J. Biochem., 1971, 19, 176–183 CrossRef.
- A. H. Good, Z. Ovary and S. J. Singer, Biochemistry, 1968, 7, 1304–1310 CrossRef CAS PubMed.
- R. M. Huseby and M. M. Murray, Biochem. Biophys. Res. Commun., 1969, 35, 169–174 CrossRef CAS PubMed.
- M. Sokolovsky and B. L. Vallee, Biochemistry, 1966, 5, 3574–3581 CrossRef CAS PubMed.
- I. Schalk, L. Ehret-Sabatier, Y. le Feuvre, S. Bon, J. Massoulie and M. Goeldner, Mol. Pharmacol., 1995, 48, 1063–1067 CAS.
- K. Minamihata, M. Goto and N. Kamiya, Bioconjugate Chem., 2011, 22, 74–81 CrossRef CAS PubMed.
- S. D. Tilley and M. B. Francis, J. Am. Chem. Soc., 2006, 128, 1080–1081 CrossRef CAS PubMed.
- M. Vilaró, G. Arsequell, G. Valencia, A. Ballesteros and J. Barluenga, Org. Lett., 2008, 10, 3243–3245 CrossRef PubMed.
- Y. Koshi, E. Nakata, M. Miyagawa, S. Tsukiji, T. Ogawa and I. Hamachi, J. Am. Chem. Soc., 2008, 130, 245–251 CrossRef CAS PubMed.
- N. S. Joshi, L. R. Whitaker and M. B. Francis, J. Am. Chem. Soc., 2004, 126, 15942–15943 CrossRef CAS PubMed.
- D. W. Romanini and M. B. Francis, Bioconjugate Chem., 2008, 19, 153–157 CrossRef CAS PubMed.
- J. M. McFarland, N. S. Joshi and M. B. Francis, J. Am. Chem. Soc., 2008, 130, 7639–7644 CrossRef CAS PubMed.
- H.-M. Guo, M. Minakawa, L. Ueno and F. Tanaka, Bioorg. Med. Chem. Lett., 2009, 19, 1210–1213 CrossRef CAS PubMed.
- M. Minakawa, H.-M. Guo and F. Tanaka, J. Org. Chem., 2008, 73, 8669–8672 CrossRef CAS PubMed.
- M. W. Jones, G. Mantovani, C. A. Blindauer, S. M. Ryan, X. Wang, D. J. Brayden and D. M. Haddleton, J. Am. Chem. Soc., 2012, 134, 7406–7413 CrossRef CAS PubMed.
- H. Ban, J. Gavrilyuk and C. F. Barbas, J. Am. Chem. Soc., 2010, 132, 1523–1525 CrossRef CAS PubMed.
- H. Ban, M. Nagano, J. Gavrilyuk, W. Hakamata, T. Inokuma and C. F. Barbas, Bioconjugate Chem., 2013, 24, 520–532 CrossRef CAS PubMed.
- D. M. Bauer, I. Ahmed, A. Vigovskaya and L. Fruk, Bioconjugate Chem., 2013, 24, 1094–1101 CrossRef CAS PubMed.
- Q.-Y. Hu, M. Allan, R. Adamo, D. Quinn, H. Zhai, G. Wu, K. Clark, J. Zhou, S. Ortiz, B. Wang, E. Danieli, S. Crotti, M. Tontini, G. Brogioni and F. Berti, Chem. Sci., 2013, 4, 3827–3832 RSC.
- J. Gavrilyuk, H. Ban, M. Nagano, W. Hakamata and C. F. Barbas, Bioconjugate Chem., 2012, 23, 2321–2328 CrossRef CAS PubMed.
- J. M. Hooker, E. W. Kovacs and M. B. Francis, J. Am. Chem. Soc., 2004, 126, 3718–3719 CrossRef CAS PubMed.
- S. J. Singer and R. F. Doolittle, Science, 1966, 153, 13–25 CAS.
- S. J. Singer, N. Martin and N. O. Thorpe, Ann. N. Y. Acad. Sci., 1971, 190, 342–351 CrossRef CAS PubMed.
- L. Wofsy, J. Kimura, D. H. Bing and D. C. Parker, Biochemistry, 1967, 6, 1981–1988 CrossRef CAS PubMed.
- T. L. Schlick, Z. Ding, E. W. Kovacs and M. B. Francis, J. Am. Chem. Soc., 2005, 127, 3718–3723 CrossRef CAS PubMed.
Footnote |
| † The numbering was generated using the Abnum program – K. R. Abhinandan et al., Molecular Immunology, 2008, 45, 3832–3839. |
| This journal is © The Royal Society of Chemistry 2015 |