Fabrication of Ag3PO4/α-Bi2O3 composites with enhanced photocatalytic properties under visible light
Feng Ding†
ab,
Sizhao Zhang†c,
Xuegang Luo*b and
Xiaoyan Linab
aSchool of Material Science and Engineering, Southwest University of Science and Technology, Mianyang 621010, Sichuan, China
bEngineering Research Center of Biomass Materials, Minister of Education, Mianyang 621010, Sichuan, China. E-mail: lxg@swust.edu.cn
cScience and Technology on Advanced Ceramic Fibers and Composites Laboratory, National University of Defense Technology, Changsha 410073, Hunan, China
First published on 29th October 2015
Abstract
The treatment of organic dyes is often conducted using the photocatalytic technique, however, the necessity for ultraviolet irradiation and efficient degradation still limit its wide practical application. Here, we report on a facile and effective co-precipitation hydrothermal method for fabricating Ag3PO4/α-Bi2O3 (AB) hierarchical heterostructures with an improved visible-light response. Rod-like α-Bi2O3 was firstly prepared under hydrothermal conditions, and then silver nitrate and disodium hydrogen phosphate were successively added to form AB. The measurement results signify that heterojunctions of polyhedral Ag3PO4 adhered to rod-like α-Bi2O3 were apparently found. AB possesses a markedly enhanced photocatalytic performance and outstanding ability in decomposing methylene blue (MB), methyl orange (MO) and rhodamine B (RhB) under visible light, compared to pure α-Bi2O3 or Ag3PO4. The amount of silver ions released reveals that the formation of α-Bi2O3 could enhance the stability of the AB composites. The fluorescence tests indicate that AB is helpful in producing the ˙OH radicals responsible for enhanced photocatalytic activity. The degradation rate could be maintained even after three degradation cycles, suggesting an admirable chemical and physical stability of AB. In addition, a possible mechanism for the enhanced photocatalytic properties of AB was also discussed.
1. Introduction
Photocatalytic degradation of organic pollutants assisted by a semiconductor has been considered to be one of the most promising approaches for solving worldwide environmental issues.1–4 Particular interest is focused on photocatalysts that function with visible light, which have wide-ranging potential applications.5,6 Most recently, it has been reported that silver orthophosphate (Ag3PO4) has extremely high photo-oxidative capabilities for O2 generation from water splitting7–9 and efficient degradation of organic dyes under visible-light irradiation,10–12 which have been paid extensive and close attention. Because of its highly dispersive conduction-band structure resulting from delocalized d states, a small effective mass of electrons was obtained, which facilitated the immigration of photoexcited electrons and holes.13 Despite the fact that Ag3PO4 displays a highly efficient visible-light photocatalytic performance, the high cost of the starting material AgNO3 and the instability upon photo-illumination prevent its wide use in the environmental and energy fields.14 It is easily corroded by photogenerated electrons (4Ag3PO4 + 6H2O + 12h+ + 12e− → 12Ag + 4H3PO4 + 3O2). If no sacrificial reagent such as AgNO3 is supplied in the system, the total Ag3PO4 photocatalyst is prone to photoreduction and decomposition during the photocatalysis process.15–17In view of the aforementioned phenomenon, extensive attention has been concentrated on the synthesis and application of coupling heterostructured materials, which could facilitate the immigration and separation of photogenerated electrons and holes, and indeed benefit the photocatalytic performance and stability. Some composites of Ag3PO4 (Ag3PO4/TiO2,18,19 carbon quantum dots (CQDs)/Ag3PO4,20 Ag/Ag3PO4,21–23 Ag3PO4/graphene,24–26 and AgX/Ag3PO4 (ref. 27 and 28)) have been prepared to overcome the disadvantages of Ag3PO4. However, construction of a high-activity heterojunction photocatalyst system is still facing a challenge as a result of the limitation of high recombination of electron–holes generated from the catalyst. It is predicted that a catalyst system with well-matched band structures may effectively handle this issue.
α-Bi2O3 has captured considerable attention due to its exceptional properties, such as a relatively narrow band gap, visible-light activity, a very stable phase at room temperature, and marked photoluminescence properties,29–31 which could result in a promising visible-light-responsive photocatalyst.32 As the valence band (VB) level of Ag3PO4 is located lower than that of α-Bi2O3, holes that are photogenerated in Ag3PO4 can transfer to α-Bi2O3, and these energetic holes in the VB of α-Bi2O3, in turn, are able to initiate various oxidation reactions. Combining Ag3PO4 with α-Bi2O3 is regarded as a potential strategy to achieve efficient photocatalysts, with high photocatalytic activities under visible-light irradiation. In more detail, a series of Ag3PO4/α-Bi2O3 photocatalysts were synthesized by hydrothermal and coprecipitation methods. The properties of the photocatalysts such as the crystal structure, morphology, and photocatalytic activity were systematically discussed including a stability assessment for the synthesized catalyst, and a mechanism of the improved photocatalytic activity of the Ag3PO4/α-Bi2O3 composite was also proposed. To the best of our knowledge, until now, no reports have been focused on the fabrication of Ag3PO4/α-Bi2O3 for dealing with the management of hazardous substances. Hence, this study may provide a rather valuable treatment method in widely degrading organic pollutants derived from modern industry in the future.
2. Experimental
2.1. Materials
Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), sodium hydroxide (NaOH), ammonia (NH3·H2O), silver nitrate (AgNO3), and disodium hydrogen phosphate (Na2HPO4) were purchased from Aladdin Reagent Co., Ltd., and were all analytical grade and used without further purification.2.2. Syntheses of pure phase α-Bi2O3
In a typical preparation process, 2 mL of polyethylene glycol, 0.4 mol of NaOH and 5 mL of NH3·H2O were added to 0.4 mol Bi(NO3)3 solutions under continuous stirring. Then, the precipitate was transferred into a Teflon-lined stainless autoclave heated at 90 °C for 2 h. Subsequently, the suspension was washed thoroughly with deionized water and was annealed at 120 °C for 4 h. At the end, the powder was calcined at 400 °C for 5 h.2.3. Preparation of Ag3PO4/α-Bi2O3 heterostructures
Ag3PO4/α-Bi2O3 (AB) heterostructures were synthesized by a co-precipitation hydrothermal method. In a typical synthesis, 50 mg of α-Bi2O3 was dispersed into 40 mL of distilled water and ultrasonically treated for 4 h. Then, 5 mmol of AgNO3 was quickly added and dissolved by stirring. Subsequently, 40 mL of 0.2 M Na2HPO4 solution was added dropwise to the solution, and a yellow precipitate appeared immediately. The resulting suspension was maintained in a 100 mL Teflon-lined stainless autoclave at 140 °C for 3 h, followed by washing the products with distilled water and absolute ethyl alcohol three times and drying at 60 °C for 10 h. The different molar ratios of the AB composites (0.1/1, 0.2/1, 0.4/1, 0.6/1, 0.8/1, and 1/1) were obtained by simply adjusting the usage of silver ions and were designated as AB-x/1, in which x refers to the molar ratio of Ag3PO4 and α-Bi2O3.2.4. Photocatalytic evaluation
Before the degradation tests, the dark absorption capacity of MB (including MO and RhB) was assessed. The dosage of AB was set at 100 mg which was added to a 250 mL glass vessel containing 100 mL of MB solution at 50 mg L−1 with magnetic stirring. The adsorption process progressed to an adsorption–desorption equilibrium after stirring the suspension for 30 min. The photocatalytic activity of the AB heterostructure was examined with regards to photodegradation of methylene blue (MB), methyl orange (MO) and rhodamine B (RhB). A 300 W Xe arc lamp with a UV cutoff filter (λ > 420 nm) was used as a light source. For the degradation tests, the photocatalyst (100 mg) was put into 100 mL of aqueous dye solution (50 mg L−1), which was magnetically stirred in the dark for 30 min to reach the adsorption equilibrium. Then, after every 5 min interval of visible-light irradiation, 3 mL of the suspension was taken and centrifuged to remove the photocatalysts for analysis. The concentrations of MB, MO and RhB were monitored by recording variations in the maximum absorption band in the UV-vis spectra.2.5. Characterizations
X-ray powder diffraction (XRD) measurements of the catalysts were conducted on a PANalytical X’Pert Pro X-ray diffractometer using Cu Kα radiation (1.54 Å), and the working voltage was 40 kV. Scanning electron microscopy (SEM) was performed with a Zeiss Ultra 55 instrument equipped with EDX along with transmission electron microscopy (TEM, Zeiss Libra 200 FE). The surface compositions of the samples were obtained by X-ray photoelectron spectroscopy (XPS, XSAM-800), using Al Kα radiation (hν = 1486.6 eV, Kratos). The UV-vis diffuse reflectance spectra of the powders were obtained using a UV-vis spectrometer (Shimadzu, UV-3150) equipped with an integrating sphere for diffuse reflectance measurements and BaSO4 was used as a reference. The optical spectra of all samples were obtained using an Ultraviolet Visible (UV-Vis) spectrometer (UV3900, Hitachi Corporation, Japan). The fluorescence spectra were obtained using a fluorescence spectrophotometer (Perkin Elmer LS-55), using 2-hydroxyterephthalic acid as a trapping agent of ˙OH. Inductively couple plasma mass spectrometry (ICP-MS, Agilent 7700x) was used for the quantification of leached silver ions.3. Results and discussion
3.1. Characterization of as-prepared catalyst
The composition and crystallographic structure of the as-prepared AB photocatalysts were determined by XRD and are shown in Fig. 1. For comparison, pure α-Bi2O3 and Ag3PO4 were also synthesized. For α-Bi2O3 (curve a in Fig. 1), all the diffraction peaks are well matched with the monoclinic Bi2O3 (JCPDS 72-0398, lattice constants a = 5.830 Å, b = 8.140 Å, c = 7.480 Å). The Ag3PO4 diffraction pattern corresponds to the body-centered cubic structure of Ag3PO4 (JCPDS 06-0505, a = b = c = 6.013 Å). As shown in Fig. 1b–g, the XRD patterns can be identified as a mixed phase of Ag3PO4 and α-Bi2O3, which rules out the possibility of other impurity phases, indicating the successful synthesis of the composites. As the amount of silver ions in the composites increases, the Ag3PO4 peaks become stronger and stronger, showing the gradual transformation of the different physical phases in the composites. | ||
| Fig. 1 XRD pattern of Ag3PO4/α-Bi2O3 composites with different molar ratios: (a) α-Bi2O3; (b) AB-0.1/1; (c) AB-0.2/1; (d) AB-0.4/1; (e) AB-0.6/1; (f) AB-0.8/1; (g) AB-1/1; (h) Ag3PO4. | ||
Fig. 2 shows typical scanning electron microscopy (SEM) images of the α-Bi2O3, Ag3PO4 and AB composites. In Fig. 2a, rod-like α-Bi2O3, with a diameter of 2 μm, and a length of 10 μm, is used as the backbone material. This result is very similar to the α-Bi2O3 prepared by other previously reported methods in the literature.33 In Fig. 2b, Ag3PO4 possessing a polyhedral morphology with an average diameter of 200 nm was observed when the mixed aqueous solution comprising AgNO3 and Na2HPO4 was hydrothermally heated in an autoclave at 140 °C for 3 h. In Fig. 2c and d, the Ag3PO4 particles are uniformly and tightly attached to the surface of α-Bi2O3, which indicates an intimate contact between α-Bi2O3 and Ag3PO4. As presented in Fig. 2e and 3a, EDS and mapping distribution show the existence of O, Bi, Ag and P, and both Ag and P are well distributed on the surface of α-Bi2O3, which further confirms the formation of an AB hierarchical structure.
 | ||
| Fig. 2 SEM images of (a) α-Bi2O3; (b) Ag3PO4; (c) AB-0.4/1; (d) AB-0.4/1 in high magnification; (e) corresponding mapping distribution of O, Bi, Ag and P. | ||
The atomic content of the constituent elements in AB-0.4/1 is listed in Table 1 taken from the red rectangle. AB-0.4/1 contained O, Bi, Ag and P with an atomic ratio of 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, which is close to the theoretical ratio of 5
24, which is close to the theoretical ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
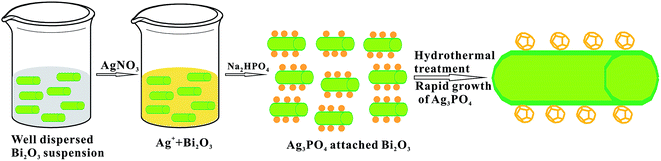
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.5. Scheme 1 illustrates the typical procedures to synthesise AB heterojunctions. Firstly, α-Bi2O3 is mixed homogeneously with H2O, but not dissolved. Secondly, AgNO3 and Na2HPO4 are successively added to the α-Bi2O3 mixture, then the PO43− reacts with the Ag+ which is evenly distributed in the α-Bi2O3 mixture to form Ag3PO4. Finally, the AB composite was heated hydrothermally at 140 °C to obtain a polyhedral morphology.
11.5. Scheme 1 illustrates the typical procedures to synthesise AB heterojunctions. Firstly, α-Bi2O3 is mixed homogeneously with H2O, but not dissolved. Secondly, AgNO3 and Na2HPO4 are successively added to the α-Bi2O3 mixture, then the PO43− reacts with the Ag+ which is evenly distributed in the α-Bi2O3 mixture to form Ag3PO4. Finally, the AB composite was heated hydrothermally at 140 °C to obtain a polyhedral morphology.
| Element | O | P | Ag | Bi |
|---|---|---|---|---|
| Content/% | 56.80 | 4.38 | 14.82 | 24.00 |
Further information about AB was obtained from transmission electron microscopy (TEM). Fig. 4a reveals that α-Bi2O3 has a diameter of 200–300 nm with a rod-like appearance and some attached particles, which is consistent with the SEM images. HRTEM images (Fig. 4b and c) confirm that there are two lattice sets with a d spacing of 0.27 and 0.36 nm, which correspond to the (120) plane of α-Bi2O3 and (211) plane of Ag3PO4, respectively. The results suggest that the heterojunction created between α-Bi2O3 and Ag3PO4 by ion exchange reactions is considered to be a well-defined crystal structure and the micro/nano structure, which has a faster charge separation, demonstrates a more efficient carrier transfer compared with pure Bi2O3 or Ag3PO4. Hence it may possess a very high photocatalytic activity in the degradation of dyes, as shown in the subsequent degradation experiments. Selected area electron diffraction (SAED) patterns could verify that AB indeed exists in the form of a single crystal, which agrees with the XRD results above.
 | ||
| Fig. 4 (a) TEM image; (b and c) high-resolution TEM images; (d) SAED pattern of AB-0.4/1 taken from the red circle. | ||
To further elucidate the elemental composition and chemical states present in the as-synthesized AB composite, XPS measurements were carried out. Fig. 5a exhibits the XPS survey spectrum of AB-0.4/1 and the peaks of P 2p, Bi 4f, C 1s, Ag 3d and O 1s can be clearly observed. In Fig. 5d, the two peaks in the spectra of Ag3PO4 appearing at 368.01 and 374.06 eV could be ascribed to the binding energies of Ag 3d5/2 and Ag 3d3/2. Peaks located in the same position are also found in the spectrum of AB-0.4/1 (Fig. 5b), accompanied by peaks at 368.01 and 374.06 eV, which are attributed to Ag+. The peaks at 132.96 (Fig. 5c) and 133.2 eV (Fig. 5e) could be attributed to P 2p in the spectra of AB-0.4/1 and Ag3PO4, respectively, which are characteristic of P5+ according to previous results.34 In Fig. 5f, the two strong peaks at 159.48 and 164.77 eV are assigned to Bi 4f7/2 and Bi 4f5/2, which correspond to Bi3+. Nonetheless, these values are not exactly the same as those obtained from pure α-Bi2O3,35,36 which indicates that an interfacial structure was formed between the AB composites and the local environment and the electron density of the elements changes to some extent. Therefore, AB can be readily synthesized by using this hydrothermal co-precipitation route, and the ratios of α-Bi2O3 and Ag3PO4 are easily controlled through adjustment of the concentration of Ag+.
The UV-vis diffuse reflectance spectra of the AB composites are displayed in Fig. 6. As shown in Fig. 6a, bare α-Bi2O3 absorbs mostly light energy in the UV region and a little of the visible area. There is an obvious enhanced absorbance in the visible-light region when Ag3PO4 is introduced into α-Bi2O3, compared with the spectrum of α-Bi2O3 alone. As Ag3PO4 is increased, the absorption in the long-wavelength range is gradually elevated. This larger absorption would lead to an improvement of the photocatalytic properties of the AB heterostructures. Band energy was calculated by the Kubelka–Munk method using the following equation:
| α(hν) = A(hν − Eg)n/2 | (1) |
 | ||
| Fig. 6 (a) UV-vis diffuse reflectance spectra of AB with different molar ratios; (b) the corresponding Kubelka–Munk transformed diffuse reflectance spectra of AB with different molar ratios. | ||
3.2. Photocatalytic performance
To ensure that the decrease in absorbance comes mainly from the photocatalytic process, a series of dark adsorption tests were conducted as seen in Fig. 7. It is notable that the adsorption capacity of MB on the different catalysts depends completely upon the catalysts, indirectly indicating that the particular surface situation of the catalysts can in some way determine the adsorption ability of the counterpart. Briefly, photocatalytic degradation experiments could not be implemented until the adsorption effects were thoroughly excluded. Put another way, the degradation rates calculated in this work are all collected after removing the influence of pure adsorption. As shown in Fig. 8, the photocatalytic activity of AB is dependent on the proportion of Ag3PO4 in the composites. The degradation rates of MB on α-Bi2O3 and AB-0.1/1 are 11.8% and 13.2% over 30 min, respectively. The relatively low photocatalytic activity of α-Bi2O3 and AB-0.1/1 is because of their low or nonexistent Ag3PO4 content. With the addition of Ag3PO4, the degradation rate is increased to 18.29% with AB-0.2/1. And the maximum value reaches 99.2% with AB-0.4/1, which is better than that of Ag3PO4. It is known that during photocatalysis, the light adsorption, and the charge transportation are crucial factors.40 Under visible light irradiation, at a low mole ratio (0.1/1 and 0.2/1), the photocatalytic properties of α-Bi2O3 are improved by the introduced Ag3PO4, which is a good visible photocatalyst and has a good light absorption in the visible region. When the AB composite mole ratio increases to 0.4/1, the photocatalytic properties of the heterostructures decrease with the increased amount of Ag3PO4. This is likely caused by excessive Ag3PO4 covering the active sites of α-Bi2O3, which hinders the electron transfer on the interface of the AB heterostructures, and thus in turn inhibits photocatalytic activity. | ||
| Fig. 7 The adsorption capacities of various dyes on corresponding catalysts after experiencing an adsorption–desorption equilibrium. | ||
 | ||
| Fig. 8 (a) Photocatalytic degradation of MB under visible light irradiation on AB; (b) photocatalytic degradation of MO and RhB on AB-0.4/1 and Ag3PO4. | ||
In additional to the MB removal, MO and RhB were also chosen as representative model dyes to evaluate the photocatalytic activity of AB. It is know that MB and RhB are cationic dyes, and MO is an anionic dye. As seen in Fig. 8a and b, the degradation rate is higher for MB and RhB than for MO. This phenomenon may be attributed to the evidence that the efficient electron transfer process hinders the recombination between the excited electrons and the produced MO+ or MB+ radicals,41 which is responsible for the enhanced photocatalytic performance of the composite catalysts under visible light irradiation. Another reason is that the cationic dyes (MB and RhB) could be easily absorbed onto the catalyst surface, which is beneficial for the adsorption content and the photocharge transfer. However, the anionic dye (MO) is not operative in this effect. In more detail, interestingly, it is also observed that the adsorption capacity of MB on both AB-0.4/1 and Ag3PO4 is nearly identical to that of RhB on those (also see the signs (1–4) in Fig. 7). But the adsorption ability (the signs (5–6) in Fig. 7) of MO on AB-0.4/1 and Ag3PO4 is substantially different from MB or RhB, and this phenomenon may be ascribed to the physical and chemical properties of the dyes, primarily referring to their cationic or anionic characteristics. Besides, the results above indicate that the surfaces of these two catalysts probably present a negative charge. Ultimately, the higher adsorption content leads to a better degradation rate for MB/RhB and MO. Thus AB is better in deteriorating the cationic dyes than the anionic dyes with regards to degradation rate.
To examine the chemical stability of the catalysts, the amount of silver ions released was also investigated. Fig. 9 shows the released silver ion concentration during the photodegradation process. As we can see, more silver ions are released from Ag3PO4 compared to the AB catalyst. These results indicate that the bare Ag3PO4 photocatalyst is not stable, and it could lose its activity under visible light irradiation. The evidence implies that the photo-excited electrons of Ag3PO4 could decompose the photocatalyst if no sacrificial reagent participates in the system. In conclusion, the combination of Ag3PO4 and α-Bi2O3 could enhance the stability of Ag3PO4.
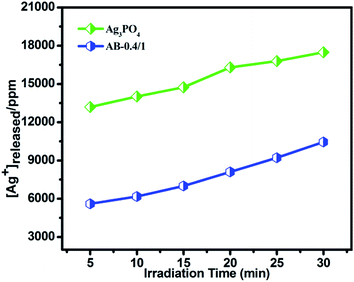 | ||
| Fig. 9 The leaching of silver ions from AB-0.4/1 and Ag3PO4 at different irradiation time during the degradation of MB. | ||
To test for ˙OH radicals, we employed a fluorescence approach based on a terephthalic acid (TA) reaction, which is frequently used in photocatalysis investigations.42 The fluorescence emission spectrum (excitation at 315 nm) of the solution was measured every 5 min during illumination. As seen in Scheme 2, ˙OH radicals with TA form 2-hydroxyterephthalic acid (TAOH) that exhibits a characteristic blue fluorescence at 425 nm. In Fig. 10, a stronger fluorescence intensity is observed for AB-0.4/1 compared to α-Bi2O3 or Ag3PO4. Because the observed fluorescence spectra were identical to that of TAOH, it is concluded that TAOH was generated from TA by reaction with ˙OH formed during photocatalysis. These results suggest that the AB hierarchical structure is helpful in producing ˙OH radicals and favorable for enhanced photocatalytic activity.
 | ||
| Fig. 10 Fluorescence spectra of terephthalic acid, α-Bi2O3, Ag3PO4 and AB-0.4/1, containing 5 mM terephthalic acid for 30 min under visible light irradiation. | ||
3.3. Mechanism of enhanced photocatalytic process
AB showed excellent photocatalytic activity with MB, RhB, and MO. The enhancement of AB composites for degradation of organic pollutants compared to bare Ag3PO4 or α-Bi2O3 photocatalysts, could be attributed to the interlaced band structure between Ag3PO4 and α-Bi2O3. We wished to further clarify the enhanced photocatalytic activity and the possible reaction mechanism (what active species were primarily involved in the degradation reactions). Reactive oxygen species trapping tests were designed to study the main reactive oxygen species in the photocatalytic process of AB-0.4/1. In this case, 1 mM EDTA as a hole scavenger was added to the MB solution to evaluate the change of the degradation rate. Eventually, a noticeable change was found in the degradation rate of MB as shown in Fig. 11 (the upmost line) compared with the control group in Fig. 11 (the lowest line), indicating that holes are the major reactive active species in the photocatalytic process. After that, benzoquinone as an O2˙− radical scavenger was also measured under similar conditions. The results show that the photocatalytic degradation rate of MB was suppressed by the addition of benzoquinone as seen in Fig. 11 (the middle line), suggesting that O2˙− radicals play a moderate role in MB degradation using AB-0.4/1 as well. | ||
| Fig. 11 Photocatalytic degradation of MB by AB-0.4/1 under different solutions (experimental conditions here are the same as those of the previous degradation process). | ||
On the basis of the characterization and photocatalytic data above, a likely mechanism for the photocatalytic degradation by AB-0.4/1 is proposed below and visualized in Fig. 12. Under visible light irradiation, α-Bi2O3 is excited to generate photogenerated electron–hole pairs, and the electrons at the valence band (VB) are excited to the conduction band (CB), inducing the formation of holes in the VB. Since the loaded Ag3PO4 is in intimate contact with α-Bi2O3, photoexcited holes from the VB of α-Bi2O3 would migrate to the less positive VB of Ag3PO4, resulting in the production of a great deal of ˙OH radicals from H2O molecules. Additionally, by the photoexcited electron-mediated reduction of O2 molecules adsorbed on the α-Bi2O3 catalyst surface, O2˙− radicals are also generated in the CB. Finally, both ˙OH and O2˙− radicals participate in effectively degrading organic dyes (MB, RhB, and MO) under visible light. In other words, the catalyst loaded with Ag3PO4 could indeed provide additional active sites for the photocatalytic process. To conclude, the evidence above implies that AB with special hierarchical heterostructures, hierarchical micro/nanostructures and favorable band structures is able to contribute strongly to accelerating electron–hole separation and enhancing the photocatalytic ability to decompose water pollutants.
3.4. Practical recycling evaluation
The stability of the catalyst is always an imperative consideration because of the importance for long-term utilization. Hence repeatability experiments were carried out to investigate the reusable properties of AB-0.4/1 in the photocatalytic process. As shown in Fig. 13, the degradation rate of MB demonstrates a high photocatalytic activity (up to 94%) even after three recycling runs, implying that the AB-0.4/1 catalyst has a rather high stability under simulated sunlight conditions. The decrease of the activity could be due to a slight loss of the catalysts during the recycling reactions. The XRD results between fresh AB-0.4/1 and after three recycling runs in Fig. 14 reveal that the structure and the phase remain relatively intact in nature, and the difference between them is focused on the intensity of the corresponding peaks. In summary, the data obtained show the feasibility and effectiveness of the recovery of the photocatalyst owing to its excellent chemical stability. | ||
| Fig. 14 Physical phase change of AB-0.4/1 catalyst using XRD pattern before and after three degradation cycles. | ||
4. Conclusions
An efficient AB photocatalyst for organic dye degradation was fabricated by loading Ag3PO4 on the surface of α-Bi2O3 through a co-precipitation hydrothermal method. The photocatalytic performance of AB shows an excellent photocatalytic degradation activity compared with bare α-Bi2O3 or Ag3PO4. The degradation rate relies upon the proportion of Ag3PO4 and the optimal degradation rate of 99.2% is achieved using the AB-0.4/1 composition. In addition, the catalyst is recoverable and reusable after three cycling runs, demonstrating the good stability of AB. The superior photoreactivity of the composite may be ascribed to the unique band structures formed, namely heterojunction micro/nano structures which have the ability to the effective separation of electrons and holes.Acknowledgements
The authors gratefully acknowledge the financial support from the Key Program of National Nuclear Facility Decommissioning and Radioactive Waste Treatment (Grant No. 2014ZG6101).References
- G. P. He, C. Xing, X. Xiao, R. P. Hu, X. X. Zuo and J. M. Nan, Appl. Catal., B, 2015, 170–171, 1–9 CAS.
- J. Du, X. Lai, N. Yang, J. Zhai, D. Kisailus, F. Su, D. Wang and L. Jiang, ACS Nano, 2011, 5, 590 CrossRef CAS PubMed.
- S. Wang, L. Yi, J. Halpert, X. Lai, Y. Liu, H. Cao, R. Yu, D. Wang and Y. Li, Small, 2012, 8, 265 CrossRef CAS PubMed.
- X. G. Luo, S. Z. Zhang, F. Ding and X. Y. Lin, Phys. Chem. Chem. Phys., 2015, 17, 22272–22285 RSC.
- H. Tong, S. X. Ouyang, Y. P. Bi, N. Umezawa, M. Oshikiri and J. H. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- A. Kubacka, M. Fernandez-Garcia and G. Colon, Chem. Rev., 2012, 112, 1555–1614 CrossRef CAS PubMed.
- Z. G. Yi, J. H. Ye, N. Kikugawa, T. Kako, S. X. Ouyang, H. Stuart-Williams, H. Yang, J. Y. Cao, W. J. Luo, Z. S. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559–564 CrossRef CAS PubMed.
- H. Zhang, H. Huang, H. Ming, H. Li, L. Zhang, Y. Liu and Z. Kang, J. Mater. Chem., 2012, 22, 10501–10506 RSC.
- Y. Bi, S. Ouyang, J. Cao and J. Ye, Phys. Chem. Chem. Phys., 2011, 13, 10071 RSC.
- Y. P. Bi, S. X. Ouyang, N. Umezawa, J. Y. Cao and J. H. Ye, J. Am. Chem. Soc., 2011, 133, 6490–6492 CrossRef CAS PubMed.
- C. T. Dinh, T. D. Nguyen, F. Kleitz and T. O. Do, Chem. Commun., 2011, 47, 7797–7799 RSC.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490–6492 CrossRef CAS PubMed.
- Q. H. Liang, W. J. Ma, Y. Shi, Z. Li and X. M. Yang, CrystEngComm, 2012, 14, 2966–2973 RSC.
- X. F. Yang, H. Y. Cui, Y. Li, J. L. Qin, R. X. Zhang and H. Tang, ACS Catal., 2013, 3, 363–369 CrossRef CAS.
- M. Ge, N. Zhu, Y. Zhao, J. Li and L. Liu, Ind. Eng. Chem. Res., 2012, 51, 5167–5173 CrossRef CAS.
- W. F. Yao, B. Zhang, C. P. Huang, C. Ma, X. L. Song and J. Q. Xu, J. Mater. Chem., 2012, 22, 4050–4055 RSC.
- H. Wang, Y. Bai, J. Yang, X. Lang and J. Li, et al., Chem.–Eur. J., 2012, 18, 5524–5529 CrossRef CAS PubMed.
- X. Ma, B. Lu, R. Shi, C. Pan and Y. Zhu, J. Phys. Chem. C, 2011, 115, 4680 CAS.
- S. B. Rawal, S. D. Sung and W. I. Lee, Catal. Commun., 2012, 17, 131–135 CrossRef CAS.
- H. C. Zhang, H. Huang, H. Ming, H. T. Li, L. L. Zhang, Y. Liu and Z. H. Kang, J. Mater. Chem., 2012, 22, 10501–10506 RSC.
- Y. P. Liu, L. Fang, H. D. Lu, Y. W. Li, C. Z. Hu and H. G. Yu, Appl. Catal., B, 2012, 115, 245–252 CrossRef.
- Y. P. Bi, H. Y. Hu, S. X. Ouyang, Z. B. Jiao, G. X. Lu and J. H. Ye, J. Mater. Chem., 2012, 22, 14847–14850 RSC.
- Y. P. Liu, L. Fang, H. D. Lu, L. J. Liu, H. Wang and C. Z. Hu, Catal. Commun., 2012, 17, 200–204 CrossRef CAS.
- H. Y. Cui, X. F. Yang, Q. X. Gao, H. Liu, Y. Li, H. Tang, R. X. Zhang, J. L. Qin and X. H. Yan, Mater. Lett., 2013, 93, 28–31 CrossRef CAS.
- X. F. Yang, H. Y. Cui, Y. Li, J. L. Qin, R. X. Zhang and H. Tang, ACS Catal., 2013, 3, 363–369 CrossRef CAS.
- G. D. Chen, M. Sun, Q. Wei, Y. F. Zhang, B. C. Zhu and B. Du, J. Hazard. Mater., 2013, 244, 86–93 CrossRef PubMed.
- Y. P. Bi, S. X. Ouyang, J. Y. Cao and J. H. Ye, Phys. Chem. Chem. Phys., 2011, 13, 10071–10075 RSC.
- J. Cao, B. D. Luo, H. L. Lin, B. Y. Xu and S. F. Chen, J. Hazard. Mater., 2012, 217–218, 107–115 CrossRef CAS PubMed.
- F. Zheng, G. Li, Y. Ou, Z. Wang, C. Su and Y. Tong, Chem. Commun., 2010, 46, 5021–5023 RSC.
- M. Faisal, A. A. Ibrahim, H. Bouzid, S. A. Al-Sayari, M. S. Al-Assiri and A. A. Ismail, J. Mol. Catal. A: Chem., 2014, 387, 69–75 CrossRef CAS.
- R. P. Hu, X. Xiao, S. H. Tu, X. X. Zuo and J. M. Nan, Appl. Catal., B, 2015, 163, 510–519 CrossRef CAS.
- H. Y. Jiang, G. G. Liu, M. Li, J. J. Liu, W. B. Sun, J. H. Ye and J. Lin, Appl. Catal., B, 2015, 163, 267–276 CrossRef CAS.
- S. Anandan, G. J. Lee, P. K. Chen, C. Fan and J. J. Wu, Ind. Eng. Chem. Res., 2010, 49, 9729–9737 CrossRef CAS.
- R. Y. Zheng, L. Lin, J. L. Xie, Y. Z. Zhu and Y. C. Xie, J. Phys. Chem. C, 2008, 112, 15502–15509 CAS.
- Z. Bian, J. Zhu, S. Wang, Y. Cao, X. Qian and H. Li, J. Phys. Chem. C, 2008, 112, 6258–6262 CAS.
- S. Shamaila, A. K. L. Sajjad, F. Chen and J. Zhang, Appl. Catal., B, 2010, 94, 272–280 CrossRef CAS.
- A. Kubacka, M. F. Garcia and G. Colon, Chem. Rev., 2011, 112, 1555–1614 CrossRef PubMed.
- X. Xiao, J. Jiang and L. Zhang, Appl. Catal., B, 2013, 142–143, 487–493 CrossRef CAS.
- C. T. Dinh, T. Nguyen, F. Kleitz and T. Do, Chem. Commun., 2011, 47, 7797–7799 RSC.
- X. J. Liu, L. K. Pan, T. Lv, Z. Sun and C. Q. Sun, J. Colloid Interface Sci., 2013, 408, 145–150 CrossRef CAS PubMed.
- L. Ge, J. Mol. Catal. A: Chem., 2008, 282, 62–66 CrossRef CAS.
- J. W. Xu, Z. D. Gao, K. Han, Y. M. Liu and Y. Y. Song, ACS Appl. Mater. Interfaces, 2014, 6, 15122–15131 CAS.
Footnote |
| † These authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2015 |