T1-weighted and T2-weighted MRI probe based on Gd-DTPA surface conjugated SPIO nanomicelles†
Waiou Zhaob,
Hailong Huanga,
Yuan Suna,
Xiaonan Zhangb,
Yapeng Li*a and
Jingyuan Wanga
aAlan G. MacDiarmid Institute of Jilin University, 2699 Qianjin Street, Changchun, China. E-mail: liyapeng@jlu.edu.cn; Fax: +86 431 85168238; Tel: +86 431 85168238
bThe First Hospital of Jilin University, Changchun, China
First published on 4th November 2015
Abstract
Herein we report novel gadolinium chelate surface conjugated superparamagnetic iron oxide (SPIO) nanomicelles. In our design, Gd-diethylenetriaminepentaacetic acid (Gd-DTPA) was conjugated with folic acid (FA) targeting the polyaspartic derivative (PASPD) coating surface of SPIO nanoparticles (IO-PASPD). The morphology of Gd-DTPA-IO-PASPD was uniformly spherical with an average particle size of 50 nm measured using dynamic light scattering (DLS) and transmission electron microscopy (TEM) imaging. The stability results showed that the FA-Gd-DTPA-IO-PASPD can stably preserve IO under physiological conditions (pH 7.4). An MTT assay showed that the cytotoxicity of FA-Gd-DTPA-IO-PASPD against hepatoma carcinoma (HepG2) cells was not significant after 24 h incubation. MRI in vitro and in vivo effects were also imaged and characterized. In summary, FA-Gd-DTPA-IO-PASPD could achieve T1-weighted and T2-weighted MR imaging simultaneously and lengthen the half life time.
Introduction
Magnetic resonance imaging (MRI) has been recognized as a powerful noninvasive diagnostic technique to visualize the fine structure of human bodies with high spatial resolution, which results from the perturbation of tissue water protons in the presence of an external magnetic field.1,2 An MR contrast agent could create good images for diagnosis by shortening the longitudinal relaxation time (T1) or the traverse relaxation time (T2) of the surrounding water protons.3,4 Paramagnetic gadolinium chelates, which create an increase in T1-weighted signal intensity (bright signal), have been demonstrated to be T1-weighted contrast (positive contrast) agents.5 On the other hand, superparamagnetic iron oxide nanoparticles (SPIONPs) with polymeric coating, which could decrease the T2-weighted MR signal (dark signal), could be demonstrated to be T2-weighted contrast (negative contrast) agents.6,7As we all know, gadolinium is often used clinically as a chelate, for example Gd-diethylenetriaminepentaacetic acid (DTPA) and Gd-1,4,7,10-tetraazacyclododecane-1,4,7,10-triacetic acid (DOTA).8–10 However, low-molecular weight Gd chelates rapidly diffuse out of the blood following administration in the vein, resulting in a low half-life,11,12 while the negative contrast showed a longer half-life in spite of the fact that iron oxide nanoparticles are often confused with a low-level MR signal arising from adjacent tissues such as bone or vasculature.13,14 In order to improve the sensitivity and specificity of lesion detection, several studies of dual-contrast (DC) MR imaging involving the administration of SPIO and gadolinium chelates have been described.15,16 Although this technique has been demonstrated to be able to improve the detection of hepatocellular carcinoma (HCC) nodules,17,18 it is still restricted by a short detection time, resulting from the short half-life of gadolinium chelates.
To overcome the disadvantages above, a dual-mode MR contrast agent prepared with gadolinium-encapsulating iron oxide has been designed and prepared.19 The large magnetic moment of the iron oxide core would create strong magnetic susceptibility in the proximity of the iron oxide core that will affect the T1 relaxation of the Gd-DTPA encapsulated within the polymeric coating of the nanoparticle. The quenching of the T1 signal failed in T1-weighted imaging of the dual-mode MR contrast. Kataoka et al. have proven that the conjugation of Gd-DTPA directly on the nanoparticle surface would not result in the quenching of the T1 values because of the greater distance between Gd-DTPA and the iron oxide core.9 Therefore, an optimal dual-MR imaging contrast would be made using gadolinium chelate surface conjugated SPIO nanomicelles, to achieve T1-weighted and T2-weighted MR imaging simultaneously and lengthen the half life time.
In this study, a new folic acid-targeted dual-mode MR contrast agent has been designed, synthesised and characterized. The designed MR agent is composed of the Gd-DTPA surface conjugated polyaspartic acid derivative (PASPD) coating of SPIO. The prepared dual-MR imaging contrast agent was carefully characterized and its stability, toxicity and paramagnetic relaxivity were evaluated. The T1-weighted and T2-weighted MRI in vivo effects were also evaluated.
Experimental
Materials
L-ASP (L-aspartic acid), diethylenetriaminepentaacetic acid (DTPA) dianhydride, gadolinium(III) chloride hexahydrate, folic acid (FA), α-methoxy-ω-amino-poly(ethylene glycol) (Mn = 2000) (MeO-PEG2000-NH2) and 1,ω-diaminopolyoxyethylene (H2N-PEG2000-NH2) were obtained from Aldrich. 85% phosphoric acid, dodecylamine (DDA), and ethylenediamine (EDA) (Beijing Chemical works) were commercially available and used without further purification. N,N-Dimethyl-formamide (DMF), and dimethylsulfoxide (DMSO) were purchased from Beijing Chemical works (China) with further purification. Human liver cancer cells HepG2 were obtained from the second hospital of Jilin University (Changchun, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) from Beijing CellChip Biotechnology Co. Ltd. IONPs were purchased from Ocean NanoTech. (SOR-10, 10 nm, oleic acid coating). Male ICR mice (6–7 weeks old) were purchased from the experimental animal center of Jilin University.Characterization
The molecular weight and polydispersity of the polymers (Mw/Mn; Mw = weight-average molecular weight; Mn = number-average molecular weight) were recorded using a gel permeation chromatography (GPC) instrument equipped with two mixed-B columns (pore size = 10 μm; column size = 300 × 7.5 mm) and a refractive index detector (Perkin-ElmerSeries 200) using DMF (0.01 mol L−1 LiBr) as the eluent at 30 °C with a flow rate of 1 mL min−1. The column system was calibrated using a set of mono-dispersed standard polystyrenes. 1H-NMR spectra were obtained using a 500 Bruker NMR instrument using CDCl3, DMSO-d6 as the solvent and TMS as the reference standard for chemical shifts.Hydrodynamic diameters (HDs) and the size distribution of polymer micelles and conjugate micelles were determined via dynamic light scattering (DLS) using a Brookheaven BI9000AT system, Brookheaven Instruments Corporation, USA, with reproducibility verified through the collection and comparison of sequential measurements. The shell model was chosen as the test mode. Measurements were performed at a 90° scattering angle at 25 °C. Each sample was measured in triplicate with 7 runs in each measurement and 90 s duration for each run. TEM imaging was used to show the size and the distribution of conjugate micelles (JEOL-2000, Japan). An MTT assay was measured using BioTek Elx 800 at a wavelength of 490 nm. The Gd metal content was determined using an inductively coupled argon plasma optical emission spectrometer (ICP-OES) (Optima 3100XL, USA).
Synthesis and characterization of FA-PASPD@IO
FA-PASPD, which has a hydrophilic segment and a hydrophobic segment, can form micelles easily. The hydrophobic–hydrophobic interaction between organic soluble IONPs and the hydrophobic DDA segments of FA-PASPD makes the IONPs water soluble. The novel IONPs can be used as MRI contrast agents. FA-PASPD (100 mg) was dissolved in 2 mL of CHCl3, and 25 mg of IONPs was dissolved in 2 mL CHCl3, too. Deionized water (10 mL) was added drop by drop to the chloroform solution under an ultrasonic wave. Chloroform was then gradually removed using rotary evaporation.Synthesis and characterization of Gd-DTPA-FA-PASPD@IO
DTPA-dianhydride (71.6 mg, 0.2 mmol) was added in portions over a period of 30 min into a solution of FA-PASPD-IO (12.5 mg, ∼0.046 mmol [NH2]) in 2 mL of 0.1 M NaHCO3. The pH of the reaction solution was adjusted to 8 by adding aliquots of a 0.1 N NaOH solution. After being stirred at room temperature for 2 h, the reaction mixture was dialyzed against PBS and deionized water (MWCO 8000). The resulting solution was concentrated to 1 mL and stored at 4 °C for future use. The amount of DTPA attached to FA-PASPD-IO was determined by quantifying the unreacted amino groups with the fluorescent amine method. Approximately 52% of the amino groups in FA-PASPD were conjugated to DTPA.To chelate with Gd3+, a solution of GdCl3·6H2O in water (100 mg mL−1) was added dropwise to the aqueous solution of DTPA-FA-PASPD-IO. The presence of trace amounts of unchelated Gd3+ in the mixture was monitored with the Gd3+ indicator 4-(2-pyridylazo) resorcinol. The reaction solution was dialyzed against water (MWCO 8000) until no free gadolinium was detected in the receiving medium. The compound contained 16.7% (w/w) of gadolinium as determined via elemental analysis. The obtained Gd-DTPA-FA-PASPD@IO particles (with a molar ratio of Gd/Fe = 0.25/1.00) were stored at 4 °C before use.
Stability of the dual-MR probe
To evaluate the stability of the NPs here, the NPs (0.2 mL, 10 mg mL−1) were incubated in PBS buffer (2 mL, 1×) at room temperature and the hydrodynamic diameters were measured at definite time intervals.Cytotoxicity assay
The cytotoxicity of the NPs against HepG2 cells was measured using an MTT assay. Experiments were conducted in triplicate. The cells were plated in 96-well plates (2 × 104 cells per well), maintained in 100 μL RPMI 1640 medium supplemented with 10% FBS and incubated for 24 h at 37 °C in a humidified atmosphere with 5% CO2. After preincubation, the NPs with pre-defined concentrations ranging from 0.01 mg mL−1 to 0.5 mg mL−1 were added into the cell culture medium. After 24 h incubation, 20 μL of MTT (5 mg mL−1 in PBS) was added to each well and the cells were incubated at 37 °C. After an incubation period of 4 h, the medium was removed and the blue formazan crystals formed inside the cells were dissolved in 100 μL of DMSO. The absorbance was measured using a BioTek Elx 800 reader at a wavelength of 490 nm.MRI in vitro
The targeting conjugate@IONPs with various iron concentrations were suspended in 0.5 mL tubes. The tubes were embedded in a home-made tank, which was designed to fit the MRI coil. T1 and T2-weighted MRI images were acquired on the MR scanner cited above with the following parameters: TR 3000 ms; TE 8, 10, 20, 40, 80, 100, 120, 140 ms; and a slice thickness of 1 mm.MRI in vivo
The experimental protocol was approved by the Animal Studies committee of Jilin University. For MR imaging, ICR male mice (6–7 weeks) were implanted with HepG2 cells (1 × 106 cells) through subcutaneous injection. When the tumors had grown to a volume of 200–500 mm3, MRI experiments were performed according to the method reported by Lee et al.22 Briefly, MR images were taken prior to injection of the NPs and at appropriate intervals post-injection. The mice received general drug anaesthesia. 0.2 mL of the dual-contrast agent (0.004 mmol Gd per mL, 0.028 mmol Gd per kg, 0.016 mmol Fe per mL, 0.110 mmol Fe per kg) was injected through the tail vein. MRI was performed using a 1.5 T Siemens Avanto MR scanner and an animal coil. All MRI quantitative analyses were carried out by a radiologist. The MRI images were exported as colour maps to be investigated and evaluated more easily. Furthermore, in order to achieve quantitative analysis, a statistical study was performed to exactly calculate the size of the colour region in the tumor site.Results and discussion
Synthesis and characterization of PASPD
The synthetic route for FA-PASPD is illustrated in Scheme 1. The detailed experiments are described in the ESI.† FA-PEG-NH2 was initially prepared by activating the γ-carboxyl group in folic acid with EDC/NHS for conjugation to the primary amine groups of PEG-diamine.20 And then, PASP-g-(PEG-FA)-DDA-EDA (FA-PASPD) was synthesized through ring-opening of PSI with FA-PEG-NH2, DDA and EDA in DMF. To demonstrate the tumor targeting property of FA, PASP-g-PEG-DDA-EDA was also synthesized according to our previous work for comparison.21 As described in our previous work, the 1H-NMR spectrum of PSI shows the signal for the methyne proton (–CH–) of the repeating succinimide unit (δ = 5.3 ppm).21 After the reaction of PSI with MeO-PEG2000-NH2, the 1H-NMR spectrum of PSI-g-PEG shows the signal for the methyne proton (–CH–) of the repeating succinimide unit after ring opening using PEG (δ = 4.6 ppm). The typical signals of PEG also can be observed at 3.5 ppm. According to the ratio of the peak area at 4.6 ppm to that at 5.3 ppm, the molar percentage of the ring-opened succinimide groups in PSI using MeO-PEG2000-NH2 is calculated to be 30%. Similarly, the molar percentage of ring-opened succinimide groups in PSI using FA-PEG-NH2 is also calculated to be 30% (Fig. S1A†). The aromatic folate proton peaks could be observed at δ 6.8–8.6 ppm. After the reaction of DDA (30% mol ratio of succinimide units), as shown in Fig. S2B,† the signals that originated from the dodecylamine units were observed at 0.88 and 1.25 ppm. From the ratio of the peak area at 4.6 ppm to that at 5.3 ppm, the molar percentage of the ring opened succinimide groups in PSI using PEG and DDA is calculated to be 60%, suggesting that a 30% molar percentage of the succinimide groups in PSI was opened using DDA. After the reaction of EDA, as shown in Fig. S1C,† the signal for the methyne proton (–CH–) of the repeating succinimide unit at 5.3 ppm completely disappeared, indicating that all residual succinimide units had reacted with EDA. The 1H-NMR spectrum of PASP-g-PEG-DDA-EDA is also given in Fig. S1D† for comparison.Characterization of Gd-DTPA-FA-PASPD-IO
The synthetic mechanism of Gd-DTPA-FA-PASPD-IO is given in Scheme 2. FA-PASPD, which has a hydrophilic segment (PEG) and a hydrophobic segment (DDA), can form micelles easily. The hydrophobic–hydrophobic interaction between organic soluble IONPs and the hydrophobic DDA segments of PASPD makes the IONPs water-soluble. The surface amine groups of FA-PASPD-IO could be functionalized with DTPA, and then the DTPA-functionalized IONPs could load the Gd3+ ions. TEM micrographs of IO, FA-PASPD-IO, PASPD-IO, Gd-DTPA-FA-PASPD-IO and Gd-DTPA-PASPD-IO are shown in Fig. 1. The content of IO in PASPD-IO and FA-PASP-IO was also measured via TGA analysis. The compound contained gadolinium as determined by elemental analysis. All of the results are shown in Table 1. | ||
| Fig. 1 TEM images of (A) IONPs, (B) PASPD-IO, (C) FA-PASPD-IO, (D) Gd-DTPA-PASPD-IO, and (E) Gd-DTPA-FA-PASPD-IO. | ||
| Nanoparticles | Dispersed in | Diameter | Fe content (mmol mL−1) | Gd content (mmol mL−1) | |
|---|---|---|---|---|---|
| a The diameter determined via TEM.b The diameter determined via DLS.c The Fe content determined via TGA.d The Gd content determined via ICP. | |||||
| IO (oleic acid coating) | Hexane | ∼10a | — | — | — |
| PASPD-IO | H2O | ∼50a | 71.2b | 0.017c | — |
| FA-PASPD-IO | H2O | ∼50a | 74.8b | 0.016c | — |
| Gd-DTPA-PASPD-IO | H2O | ∼50a | 80.6b | 0.017c | 0.004d |
| Gd-DTPA-FA-PASPD-IO | H2O | ∼50a | 82.3b | 0.016c | 0.004d |
Stability analysis
The stability in biological media is critical for a contrast agent delivery system because the NPs should stay in the blood for a sufficiently long time for active recognition and to be uptaken by the target organs. To evaluate the stability of the micelles here, they were incubated in PBS buffer (pH 7.4, 1×) at 37 °C with the hydrodynamic diameters being measured at definite time intervals. As shown in Fig. 2, almost no change in the particle size was detected during the 30-day storage time. The results indicated the high stability of the conjugate micelles under physiological conditions. | ||
| Fig. 2 The hydrodynamic diameters as a function of the storage time at 4 °C for all the nanoparticles in PBS buffer (pH 7.4, 1×). | ||
MTT analysis
The in vitro cytotoxicity of Gd-DTPA-PASPD@IO should be demonstrated because dual-contrast agents must show high biocompatibility. After 24 h incubation, the effect of the dual-contrast agents with a series of concentrations on the viability of HepG2 cells was measured using the MTT assay. The cell viability results are shown in Fig. 3. None of the NPs with different concentrations showed significant cytotoxicity against the cells and 80% of the cells remained viable. The results suggested that the cytotoxicity of the dual-contrast agent was not affected by Gd-DTPA surface conjugating. Therefore, this dual-functional probe showed biocompatibility and the potential applications of our nanoprobes for the imaging of cancers. | ||
| Fig. 3 Cytotoxicity of all of the nanoparticles; the concentrations range from 0.50 mg mL−1 to 0.01 mg mL−1. | ||
MRI in vitro
To evaluate the MRI contrast effect, a phantom study was performed with IONPs at elevated concentrations. As shown in Fig. 4A, the targeting IONPs displayed a clear concentration-dependent T2 signal reduction effect, with an R2 value of 173 mM−1 s−1 (based on Fe concentration). This was likely attributed to a better magnetization control allowed by not destroying the surface of the hydrophobic IONPs. The high R2 values of the delivery system in comparison with the commercially available products render them promising as an MRI-visible drug delivery system. To evaluate the T1 reducing effect, the dual-contrast at the different Fe concentrations above was investigated. The phantom images (Fig. 4B) demonstrated that the T1 signal intensity increased significantly compared with FA-PASPD-IO. The R1 value of the dual contrast was 40.2 mM−1 s−1. Overall, the above results indicate that surface-conjugation of Gd-DTPA with the coating of the IONPs showed no quenching of Gd.MRI in vivo
T1-weighted and T2-weighted fast spin-echo images pre-injection, and 0.5 h and 2 h post-injection were acquired. As displayed in Fig. 5, a dramatic T2 signal drop was witnessed in the tumor area after the injection of FA-Gd-DTPA-IO-PASPD while a relative weak signal drop was observed in mice injected with Gd-DTPA-IO-PASPD. On the other hand, a dramatic T1 signal increase was witnessed in the tumor area after the injection of FA-Gd-DTPA-IO-PASPD while a relatively weak signal increase was observed in mice injected with Gd-DTPA-IO-PASPD. It is noteworthy that even at the 24 h time point, the enhancement of FA-Gd-DTPA-IO-PASPD in dual-mode imaging was also observed compared with Gd-DTPA-IO-PASPD, suggesting the long circulation half-life of FA-Gd-DTPA-IO-PASPD. As described above, FA-Gd-DTPA-IO-PASPD could be used as a dual-mode imaging contrast agent with a long circulation half-life. The half-life of Gd-DTPA was also prolonged.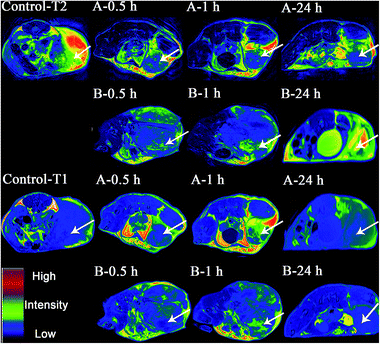 | ||
| Fig. 5 T2-weighted and T1-weighted magnetic resonance images of tumors injected with (A) Gd-DTPA-FA-PASPD-IO and (B) Gd-DTPA-PASPD-IO. The arrows denote allograft tumors. | ||
Conclusions
In summary, a novel dual-MR contrast nanoprobe, based on Gd-DTPA surface conjugated SPIO nanomicelles was reported. The results demonstrated that the probe prepared this way could achieve T1-weighted MR imaging and T2-weighted MR imaging simultaneously, lengthen the half-life time, and make receptor-targeted internalization. Meanwhile, the low cytotoxicity and high stability also proved that the nanoparticles could be used as a dual-MR probe from a practical standpoint. Overall, the FA-targeted Gd-DTPA surface conjugated SPIO nanomicelles could be a novel platform for cancer diagnosis technology.Notes and references
- M. Rudin and R. Weissleder, Drug Discovery, 2003, 2, 123 CrossRef CAS PubMed.
- R. Hao, R. Xing, Z. Xu, Y. Hou, S. Gao and S. Sun, Adv. Mater., 2010, 22, 2729 CrossRef CAS PubMed.
- X. Kang, Z. Cheng, D. Yang, P. Ma, M. Shang, C. Peng, Y. Dai and J. Lin, Adv. Mater., 2012, 22, 1470 CAS.
- X. Lu, R. Jiang, Q. Fan, L. Zhang, H. Zhang, M. Yang, Y. Ma, L. Wang and W. Huang, J. Mater. Chem., 2012, 22, 6965 RSC.
- L. Frullano and T. J. Meade, J. Biol. Inorg. Chem., 2007, 12, 939 CrossRef CAS PubMed.
- T. Chen, M. I. Shukoor, R. Wang, Z. Zhao, Q. Yuan, S. Bamrungsap, X. Xiong and W. Tan, ACS Nano, 2011, 5, 7866 CrossRef CAS PubMed.
- H. M. Kim, H. Lee, K. S. Hong, M. Y. Cho, M. H. Sung, H. Poo and Y. T. Lim, ACS Nano, 2011, 5, 8230 CrossRef CAS PubMed.
- T. P. Gazzi, L. A. Basso, D. S. Santos and P. Machado, RSC Adv., 2014, 4, 9880 RSC.
- P. Mi, D. Kokuryo, H. Cabral, M. Kumagai, T. Nomotoa, I. Aoki, Y. Terada, A. Kishimura, N. Nishiyama and K. Kataoka, J. Controlled Release, 2014, 174, 63 CrossRef CAS PubMed.
- J. Hu, T. Liu, G. Zhang, F. Jin and S. Liu, Macromol. Rapid Commun., 2013, 34, 749 CrossRef CAS PubMed.
- K. M. Bennett, J. Jo, H. Cabral, B. Rumiana and I. Aoki, Adv. Drug Delivery Rev., 2014, 74, 75 CrossRef CAS PubMed.
- H. Ishiwata, A. Vertut-Doi, T. Hirose and K. Miyajima, Chem. Pharm. Bull., 1995, 43, 1005 CrossRef CAS PubMed.
- J. W. Bulte and D. L. Kraitchman, NMR Biomed., 2004, 17, 484 CrossRef CAS PubMed.
- J. Zhuo and R. P. Gullapalli, RadioGraphics, 2006, 26, 275 CrossRef PubMed.
- L. Macarini, S. Marini and P. Milillo, Radiol. Med., 2006, 111, 1087 CrossRef CAS PubMed.
- R. C. Semelka, J. K. Lee and S. Worawattanakul, J. Magn. Reson. Imaging, 1998, 8, 670 CrossRef CAS PubMed.
- J. Ward, J. A. Guthrie and D. J. Scott, Radiology, 2000, 216, 154 CrossRef CAS PubMed.
- N. Bolog, T. Pfammatter and B. Mullhaupt, Abdom. Imag., 2008, 33, 313 CrossRef PubMed.
- S. Santra, S. D. Jativa, C. Kaittanis, G. Normand, J. Grimm and J. M. Perez, ACS Nano, 2012, 6, 7281 CrossRef CAS PubMed.
- Y. Chang, N. Liu, L. Chen, X. Meng, Y. Liu, Y. Li and J. Wang, J. Mater. Chem., 2012, 22, 9594 RSC.
- H. Huang, Y. Li, X. Sun, Y. Lv, L. Chen and J. Wang, New J. Chem., 2013, 37, 1623 RSC.
- Y. Lee, S. Y. Park, H. Mok and T. G. Park, Bioconjugate Chem., 2008, 19, 525 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18165h |
| This journal is © The Royal Society of Chemistry 2015 |



