Bismuth nanoparticles: an efficient catalyst for reductive coupling of nitroarenes to azo-compounds†
Kishore Pothulaab,
Lin Tangab,
Zhenggen Zhaab and
Zhiyong Wang*ab
aHefei National Laboratory for Physical Sciences at Microscale, CAS Key Laboratory of Soft Matter Chemistry & Collaborative Innovation Center of Suzhou Nano Science and Technology, P. R. China. E-mail: zwang3@ustc.edu.cn; Fax: +86 551 360 3185
bDepartment of Chemistry, University of Science and Technology of China, Hefei, 230026, P. R. China
First published on 16th September 2015
Abstract
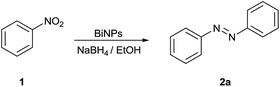
The synthesis of azoarenes from corresponding nitroarenes was developed by virtue of in situ bismuth nanoparticles. A series of aromatic azo compounds can be obtained under mild reaction conditions with excellent yields.
Rather than carbon nanostructures, in recent years research focused on the controlled synthesis and study of the nano-structures/properties of transition metals and binary alloys, such as Au, Ag, Pt, Pd, Co, and Pt/Fe, has been proven to be successful.1 In contrast, the syntheses of main group metal nanoparticles are relatively not studied much. Bismuth is the heaviest main group element and it is an abundant, inexpensive and non-toxic material. In addition, bulk bismuth exhibits semimetal behaviour with a small band overlap and an anisotropic electron effective mass. The above unique and special characteristics of bismuth encouraged researchers to focus on its nano-nature followed by studying its applications. Previous studies have found that as the size decreases bismuth could transfer from a semimetal to a semiconductor.2 Over the past two decades, bismuth nanostructures such as thin films, nanowires, nanolines, nanobelts, nanopipes, nanotubes and others3 have been extensively studied.
Though extensive research has been carried out on the physical characteristics such as the semiconductor and thermoelectric nature of bismuth,4 much interest has not yet been directed on the exploration of its chemical properties such as employing bismuth nanoparticles as a catalyst for effective chemical conversions. Our research group is interested in studying “nano-metals and exploring their applications in organic synthesis”. To address the above aim, we have taken up the current objectives of (i) synthesis of bismuth nanoparticles (BiNPs) from the reported literature5 with some modifications and (ii) evaluation of their catalytic property in the chemoselective conversion of aryl nitro to aryl azo compounds.
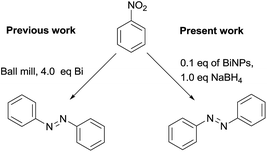
“Azo” is one of the very important organic functional groups and in particular aromatic azo-compounds have wide applications in academic and industrial research, such as radical reaction initiators, therapeutic and drug delivery agents, chelating agents, pigments, organic dyes, food additives and many others.6 Among the various methods reported in the literature, two methods are prominent for the synthesis of aromatic azo compounds, (i) coupling of diazonium salts with electron-rich aromatics7 and (ii) metal based reductive coupling of nitroaromatics by using Bi, Pt, Pd, Ru, In, Mg, Zn, Fe, Nb, Au, Sm and others.8 Both of the methods suffer from either safety related concerns or a tedious work-up process, which still need to be addressed thoroughly. Recently, oxidative coupling of arylamines into azos was also reported,9 yet it needs to be explored further. Previously azo-arenes were prepared by using stoichiometric amounts of Bi metal8a (Scheme 1), but the method was not suitable for wider applications such as academic/industrial research.
As our research group has been focusing on heterogeneous catalysis10 for a long time, herein we report an efficient method for the chemoselective preparation of aromatic azo compounds.
In a typical procedure, 0.5 mmol BiCl3 was suspended in 30 ml of demineralized water. After stirring for about 15 min at room temperature (RT, ∼25–30 °C), white precipitation was observed. Then 0.75 mmol of zinc dust was added in portions and the reaction mixture was further stirred for 1 hour. Black particles were observed in the reaction. The pH was adjusted to 3 with aqueous hydrochloric acid. The reaction mixture was filtered and washed with water, followed by ethanol and then dried under vacuum for 12 hours at 50 °C to obtain BiNPs (yield 92%). The reported procedure for BiNPs was for bamboo raft nano-tubes, here we got nano-bismuth particles at pH 3.
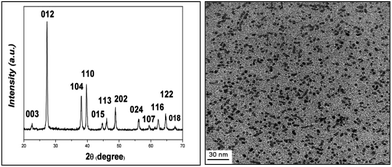
The bismuth nanoparticles were characterized by X-ray diffraction (XRD) and Transmission Electron Microscopy (TEM) techniques. Fig. 1a shows the typical powder XRD pattern of the as-synthesized bismuth nanoparticles. The morphologies and structures of the as-prepared bismuth nanoparticles were investigated by TEM images Fig. 1b. The diameters of these nanoparticles varied from 3 to 8 nm.
The catalytic activity of the bismuth nanoparticles in the reductive coupling of nitroarenes in the presence of NaBH4 as a reducing agent and ethanol as a solvent was investigated. The reaction mixture of nitrobenzene and bismuth nanoparticles was degassed and the NaBH4/ethanol solution was added followed by stirring of the reaction mixture for 24 hours. The progress of the reaction mass was monitored by TLC (mobile phase pet ether/ethyl acetate: 9/1). The catalyst was filtered and the filtrate was evaporated under reduced pressure. The obtained product was purified by column chromatography to afford pure azo-benzene.
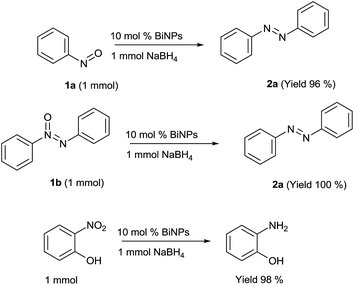
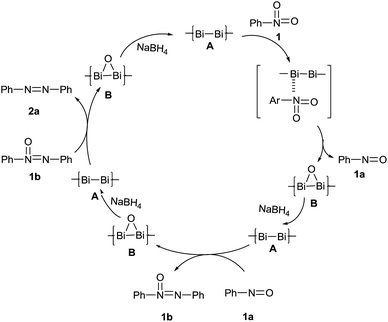
The reaction conditions were optimized by examining the influence/impact of the mole ratio of the catalyst and the reducing agent in various experiments and the results are depicted in Table 1. Apart from the above conditions, the reaction was also conducted in water at different temperatures (30, 50 and 80 °C), but the yield is less than 20% (see the ESI Table S1†). This indicates that water disfavored this reaction. During the intermediate stage of the reaction, the sample was analysed by GC-mass and it was found that the levels of nitro, nitroso, azoxy and azobenzenes were at 10, 30, 9 and 42% respectively. To prove the conceptual bias, a few control experiments (Fig. 2) were conducted by converting the respective intermediates (1a and 1b) into azo compounds at the given conditions. In terms of these experimental results, the possible mechanism is depicted in Scheme 2.
| Entry | Catalyst (equiv.) | Reductant (equiv.) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: nitrobenzene (1 mmol), catalyst BiNPs, reductant NaBH4, solvent 4 ml of ethanol, 25–30 °C, 24 h.b Yield with reference to nitrobenzene. | |||
| 1 | 1 | — | Traces |
| 2 | — | 1 | — |
| 3 | 0.5 | 0.5 | 87 |
| 4 | 0.2 | 0.5 | 80 |
| 5 | 0.2 | 1 | 92 |
| 6 | 0.1 | 1 | 92 |
| 7 | 0.05 | 1 | 65 |
| 8 | 0.05 | 1 | 68 |
| 9 | 0.05 | 2 | 68 |
The conversion of the nitro group to the nitroso group is most likely the key step of this overall transformation. First of all, nitrobenzene 1 is converted to nitrosobenzene 1a which reacts with another molecule of nitrosobenzene to give azoxybenzene 1b which is further reduced to azobenzene 2a. In each intermediate step, bismuth oxide B is formed and then reconverts to a bismuth nanoparticle A by sodium borohydrate, subsequently. Moreover, the electron donating substituent would facilitate the nucleophilic attachment of the oxygen atom of the nitro function onto the surface of the bismuth nanoparticles in a push pull manner, while the electron-withdrawing group would disfavour such process. When o-nitrophenol reacted under the conditions employed, it was readily converted to o-aminophenol (Fig. 2) instead of an azo compound. Perhaps the electron-donation of the hydroxyl substituent prompted the reduction of the nitro to an amino group while this ortho-substitution blocked the cross-coupling.
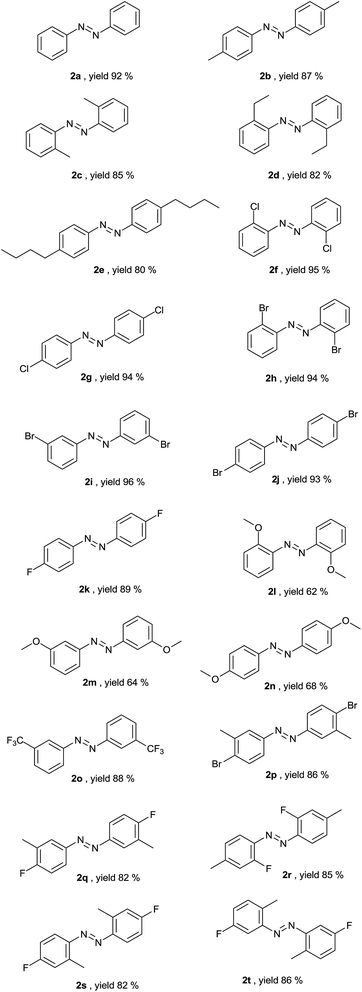
The generalization of the scheme is explained by proving its efficiency on various examples (Table 2) with diversified substitutions on the aromatic ring. The reaction is general and can be employed for the synthesis of mono-substituted halo (2f–2k), alkoxy (2l–2n), alkyl (2b–2e) and di-substituted (2p–2t) compounds. The present methodology was successfully extended to the single step synthesis of long chain bis-(4-butyl-phenyl)-diazene (2e) from 4-butyl nitrobenzene. The reaction was clean and the yield based on conversion was almost quantitative. Unsatisfactory conversion was observed with a substituted alkoxy group (yield 62–68% for compounds 2l–2n), but it may also reflect some role played by the alkoxy group in the reduction of nitroarenes on the structured solid surface. When two different nitroarenes (2-methyl nitrobenzene and 2-chloro nitrobenzene) reacted together under the given conditions, two symmetrical azoarenes were formed in an approximate ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. No unsymmetrical azoarenes were observed during the reaction. All of the compounds obtained were well characterized by 1H and 13C NMR spectra and melting points. A few unreported compounds (2r–2t) were synthesized and characterised by NMR spectra, HRMS, IR spectra and melting points together.
1. No unsymmetrical azoarenes were observed during the reaction. All of the compounds obtained were well characterized by 1H and 13C NMR spectra and melting points. A few unreported compounds (2r–2t) were synthesized and characterised by NMR spectra, HRMS, IR spectra and melting points together.
| a Reaction conditions: nitrobenzene (1 mmol), BiNPs 10 mol% (0.1 mol equiv.), NaBH4 1 mol equiv. in 4 ml of EtOH, 25 °C, 24 h. |
|---|
 |
As this is a heterogeneous catalysis reaction, the possibility of recycling the catalyst and reusability followed by its impact on yield was examined (Table 3). The yield was good (85%) even after the 5th run of bismuth nanoparticles.
| a Reaction conditions: nitrobenzene (1 mmol) in 4 ml of ethanol, BiNPs (10 mol%), sodium borohydrate (1.0 equiv.), 25–30 °C, 24 h. | |||||
|---|---|---|---|---|---|
| Cycle (run) | 1 | 2 | 3 | 4 | 5 |
| Yield (%) | 95 | 93 | 90 | 88 | 85 |
In summary a remarkable heterogeneous bismuth catalyst system was developed successfully and the application of this catalyst in the coupling of aromatic nitro compounds was achieved to afford the corresponding nitroarenes in excellent yields under environmentally benign conditions. The reaction has a broad scope of reaction substrates, and a variety of functional groups can tolerate the reaction conditions well. Moreover, the catalyst can be recovered and reused through simple phase separation. Ongoing research including further mechanistic details, expanding the substrate scope and applications in organic synthesis is currently underway.
Acknowledgements
We are grateful to National Nature Science Foundation of China (21432009, 2127222, 91213303, 21472177) and CAS-TWAS (Chinese Academy of Sciences and The Academy of Sciences for the developing world) for financial support.Notes and references
- (a) Y. J. Xiong, J. Y. Chen, B. Wiley, Y. A. Xia, Y. D. Yin and Z. Y. Li, Nano Lett., 2005, 5, 1237 CrossRef CAS; (b) W. Z. Wang, J. Y. Huang and Z. F. Ren, Langmuir, 2005, 21, 751 CrossRef CAS PubMed; (c) J. Y. Chen, B. Wiley, Z. Y. Li, D. Campbell, F. Saeki, H. Cang, L. Au, J. Lee, X. D. Li and Y. N. Xia, Adv. Mater., 2005, 17, 2255 CrossRef CAS PubMed; (d) C. J. Murphy, Science, 2002, 298, 2139 CrossRef CAS PubMed; (e) Y. G. Sun and Y. N. Xia, Science, 2002, 298, 2176 CrossRef CAS PubMed; (f) Z. L. Wang, J. Phys. Chem. B, 2000, 104, 1153 CrossRef CAS; (g) C. L. V. Haynes and R. P. Duyne, J. Phys. Chem. B, 2001, 105, 5599 CrossRef CAS; (h) X. W. Teng, D. Black, N. J. Watkins, Y. L. Gao and H. Yang, Nano Lett., 2003, 3, 261 CrossRef CAS; (i) A. G. Tkachenko, H. Xie, D. Coleman, W. Glomm, J. Ryan, M. F. Anderson, S. Franzen and D. L. Feldheim, J. Am. Chem. Soc., 2003, 125, 4700 CrossRef CAS PubMed; (j) T. Seto, H. Akinaga, F. Takano, K. Koga, T. Orii and M. Hirasawa, J. Phys. Chem. B, 2005, 109, 13403 CrossRef CAS PubMed.
- C. A. Hoffman, J. R. Meyer, F. J. Bartoli, A. Di Venere, X. J. Yi, C. L. Hou, H. C. Wang and J. B. Ketterson, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 11431 CrossRef CAS.
- (a) D. H. Kim, S. H. Lee, J. K. Kim and G. H. Lee, Appl. Surf. Sci., 2006, 252, 3525 CrossRef CAS PubMed; (b) M. S. Dresselhaus, Y. M. Lin, O. Rabin, A. S. Jorio, A. G. Filho, M. A. Pimenta, R. Saito, G. Samsonidze and G. Dresselhaus, Mater. Sci. Eng., C, 2003, 23, 129 CrossRef; (c) K. Miki, J. H. G. Owen, D. R. Bowler, G. A. D. Briggs and K. Sakamoto, Surf. Sci., 1999, 421, 397 CrossRef CAS; (d) Y. Chen, R. Gong, W. Zhang, X. Xu, Y. Fan and W. Liu, Mater. Lett., 2005, 59, 909 CrossRef CAS PubMed; (e) Y. Li, J. Wang, Z. Deng, Y. Wu, X. Sun, D. Yu and P. Yang, J. Am. Chem. Soc., 2001, 123, 9904 CrossRef CAS.
- (a) M. R. Black, Y. M. Lin, S. B. Cronin, O. Rabin and M. S. Dresselhaus, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 195417 CrossRef; (b) C. J. Tang, G. H. Li, X. C. Dou, Y. X. Zhang and L. Li, J. Phys. Chem. C, 2009, 113, 5422 CrossRef CAS.
- B. Yang, C. Li, H. Hu, X. Yang, Q. Li and Y. Qian, Eur. J. Inorg. Chem., 2003, 3699 CrossRef CAS PubMed.
- (a) A. Bafana, S. S. Devi and T. Chakrabarti, Environ. Rev., 2011, 19, 350 CrossRef CAS; (b) R. G. Anderson and G. Nickless, Analyst, 1967, 92, 207 RSC; (c) N. D. P. Ashutosh and J. K. Mehrotra, Colourage, 1979, 26, 25 CAS; (d) C. S. Sheppard, Encycl. Polym. Sci. Eng., 1985, 2, 143 CAS; (e) K. V. D. Bernaerts and F. E. Prez, Prog. Polym. Sci., 2006, 31, 671 CrossRef CAS PubMed; (f) D. M. Burland, R. D. Miller and C. A. Walsh, Chem. Rev., 1994, 94, 31 CrossRef CAS; (g) D. R. Kanis, M. A. Ratner and T. J. Marks, Chem. Rev., 1994, 94, 195 CrossRef CAS; (h) E. Ishow, C. Bellaıche, L. Bouteiller, K. Nakatani and J. A. Delaire, J. Am. Chem. Soc., 2003, 125, 15744 CrossRef CAS PubMed; (i) S. K. Yesodha, C. K. S. Pillai and N. Tsutsumi, Prog. Polym. Sci., 2004, 29, 45 CrossRef CAS PubMed; (j) F. Qiu, Y. Cao, H. Xu, Y. Jiang, Y. Zhou and J. Liu, Dyes Pigm., 2007, 75, 454 CrossRef CAS PubMed; (k) B. Sahraoui, J. Luc, A. Meghea, R. Czaplicki, J.-L. Fillaut and A. Migalska-Zalas, J. Opt. A: Pure Appl. Opt., 2009, 11, 24005 CrossRef; (l) I. Papagiannouli, K. Iliopoulos, D. Gindre, B. Sahraoui, O. Krupka, V. Smokal, A. Kolendo and S. Couris, Chem. Phys. Lett., 2012, 554, 107 CrossRef CAS PubMed; (m) M. Ashraf, A. Teshome, A. J. Kay, G. J. Gainsford, M. D. H. Bhuiyan, I. Asselberghs and K. Clays, Dyes Pigm., 2012, 95, 455 CrossRef CAS PubMed; (n) N. DiCesare and J. R. Lakowicz, Org. Lett., 2001, 3, 3891 CrossRef CAS PubMed; (o) V. K. Bhardwaj, N. Singh, M. S. Hundal and G. Hundal, Tetrahedron, 2006, 62, 7878 CrossRef CAS PubMed; (p) J. Isaad and A. Perwuelz, Tetrahedron Lett., 2010, 51, 5810 CrossRef CAS PubMed; (q) K.-C. Chang, I.-H. Su, Y. Y. Wang and W. S. Chung, Eur. J. Org. Chem., 2010, 4700 CrossRef CAS PubMed.
- (a) M. Barbero, I. Degani, S. Dughera, R. Fochi and P. Perracino, Synthesis, 1998, 1235 CrossRef CAS; (b) J. Merrington, M. James and M. Bradley, Chem. Commun., 2002, 140 RSC; (c) M. Tomasulo and F. M. Raymo, Org. Lett., 2005, 7, 4633 CrossRef CAS PubMed; (d) W.-J. Tsai, Y.-J. Shiao, S.-J. Lin, W.-F. Chiou, L.-C. Lin, T. H. Yang, C. M. Teng, T. S. Wu and L. M. Yang, Bioorg. Med. Chem. Lett., 2006, 16, 4440 CrossRef CAS PubMed; (e) M. Barbero, S. Cadamuro, S. Dughera and C. Giaveno, Eur. J. Org. Chem., 2006, 4884 CrossRef CAS PubMed; (f) M. H. Lee, B.-K. Cho, J. Yoon and J. S. Kim, Org. Lett., 2007, 9, 4515 CrossRef CAS PubMed; (g) H. A. Dabbagh, A. Teimouri and A. N. Chermahini, Dyes Pigm., 2007, 73, 239 CrossRef CAS PubMed; (h) Y. He, W. He, R. Wei, Z. Chen and X. Wang, Chem. Commun., 2012, 1036 RSC.
- (a) S. Wada, M. Urano and H. Suzuki, J. Org. Chem., 2002, 67, 8254 CrossRef CAS PubMed; (b) J. A. Gladysz, J. G. Fulcher and S. Togashi, Tetrahedron Lett., 1977, 18, 521 CrossRef; (c) M. E. Krolski, A. F. Renaldo, D. E. Rudisill and J. K. Stille, J. Org. Chem., 1988, 53, 1170 CrossRef CAS; (d) H. J. Shine and H. E. Mallory, J. Org. Chem., 1962, 27, 2390 CrossRef CAS; (e) J. M. Khurana and A. Ray, Bull. Chem. Soc. Jpn., 1996, 69, 407 CrossRef CAS; (f) C. Yu, B. Liu and L. Hu, J. Org. Chem., 2001, 66, 919 CrossRef CAS; (g) F. A. Khan and C. Sudheer, Tetrahedron Lett., 2009, 50, 3394 CrossRef CAS PubMed; (h) L. Hu, X. Cao, L. Chen, J. Zheng, J. Lu, X. Sun and H. Gu, Chem. Commun., 2012, 48, 3445 RSC; (i) N. Sakai, K. Fujii, S. Nabeshima, R. Ikeda and T. Konakahara, Chem. Commun., 2010, 46, 3173 RSC; (j) Y. Moglie, C. Vitale and G. Radivoy, Tetrahedron Lett., 2008, 49, 1828 CrossRef CAS PubMed; (k) J. Hyun Kim, J. H. Park, Y. K. Chung and K. H. Park, Adv. Synth. Catal., 2012, 354, 241 Search PubMed; (l) X. Liu, H.-Q. Li, S. Ye, Y.-M. Liu, H.-Y. He and Y. Cao, Angew. Chem., Int. Ed., 2014, 53, 7624 CrossRef CAS PubMed; (m) X. Liu, S. Ye, H.-Q. Li, Y.-M. Liu, Y. Cao and K.-N. Fan, Catal. Sci. Technol., 2013, 3, 3200 RSC; (n) H. Zhu, X. Ke, X. Yang, S. Sarina and H. Liu, Angew. Chem., Int. Ed., 2010, 49, 9657 CrossRef CAS PubMed; (o) J. Wang, L. Hu, X. Cao, J. Lu, X. Li and H. Gu, RSC Adv., 2013, 3, 4899 RSC; (p) S. H. Gund, R. S. Shelkar and J. M. Nagarkar, RSC Adv., 2014, 4, 42947 RSC.
- (a) C. Zhang and N. Jiao, Angew. Chem., Int. Ed., 2010, 49, 6174 CrossRef CAS PubMed; (b) S. Cai, H. Rong, X. Yu, X. Liu, D. Wang, W. He and Y. Li, ACS Catal., 2013, 3, 478 CrossRef CAS.
- (a) Y. Wang, D. Zhu, L. Tang, S. Wang and Z. Wang, Angew. Chem., Int. Ed., 2011, 50, 8917 CrossRef CAS PubMed; (b) L. Tang, H. Sun, Y. Li, Z. Zha and Z. Wang, Green Chem., 2012, 14, 3423 RSC; (c) L. Tang, X. Guo, Y. Li, S. Hang, Z. Zha and Z. Wang, Chem. Commun., 2013, 49, 5213 RSC; (d) X. Guo, L. Tang, Y. Yang, Z. Zha and Z. Wang, Green Chem., 2014, 16, 2443 RSC; (e) L. Tang, X. Guo, Y. Yang, Z. Zha and Z. Wang, Chem. Commun., 2014, 50, 6145 RSC; (f) L. Tang, Y. Yang, L. Wen, S. Zhang, Z. Zha and Z. Wang, Org. Chem. Front., 2015, 2, 114 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization of products. See DOI: 10.1039/c5ra17994g |
| This journal is © The Royal Society of Chemistry 2015 |