Preparation, characterization and application of antibody-conjugated magnetic nanoparticles in the purification of begomovirus
Abstract
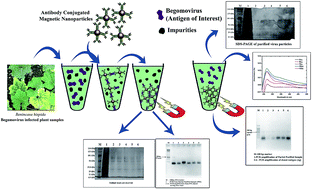
Begomovirus (family-Geminiviridae) infects a wide range of commercial crops like tomato, bean, cassava, cotton, cucurbits and chilli. Purification of begomoviruses from the infected plants, in particular from vegetable crops remains challenging. The conventional process of begomovirus purification requires sophisticated instruments and moreover, it is time-consuming. Herein, we used antibody-conjugated magnetic nanoparticles (Ab-MNPs) to purify begomoviruses from the infected plants. MNPs were prepared using the co-precipitation method (at pH between 8 and 12 & size 25 nm). The prepared MNPs were functionalized with APTES (at pH 7) and confirmed with FTIR. Thus functionalized MNPs were conjugated with polyclonal antibodies (pAbs) using EDC–NHS chemistry (size = 80 nm). The crude extract prepared from the infected plants was suspended in the solution of Ab-MNPs and separated using a magnet. The captured virus particles were released into the aqueous solution (at pH 10). SDS-PAGE analysis and PCR analysis were done to confirm the presence of viral infection.

 Please wait while we load your content...
Please wait while we load your content...