PET governed fluorescence “Turn ON” BODIPY probe for selective detection of picric acid†
Yogesh Erande,
Santosh Chemate,
Ankush More and
Nagaiyan Sekar*
Tinctorial Chemistry Group, Department of Dyestuff Technology, Institute of Chemical Technology, Mumbai-400 019, India. E-mail: n.sekar@ictmumbai.edu.in; nethi.sekar@gmail.com; Tel: +91 22 3361 2707
First published on 8th October 2015
Abstract
The non-fluorescent meso diaminophenyl 1,3,5,7-tetramethyl BODIPY dye has been investigated and employed for picric acid sensing in a constructive way by regenerating its fluorescence through PET hindrance. A strong enhanced emission signal was obtained as a consequence of the electrostatic association between the BODIPY probe and picric acid with specific recognition among other explosive nitroaromatics. The probe shows a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry with picric acid and a detection limit of up to 0.65 ppb.
1 binding stoichiometry with picric acid and a detection limit of up to 0.65 ppb.
Introduction
Nowadays the danger of terrorism with an increased use of high explosives challenges modern technology to detect and screen them.1 Electron deficient nitroaromatics (NACs) are preferred explosives and picric acid (PA) is one of them whose explosive power is equivalent to 105% of trinitrotoluene (TNT), which is known as a primary explosive.2,3 PA is an important chemical and finds applications in medicinal formulations in the treatment of malaria, trichinosis, herpes, and smallpox, and as antiseptics,4 in dye industries and chemical laboratories,5,6 in the manufacture of rocket fuels and fireworks, as sensitizers in photographic emulsions, and as a component in matches.7–9 Owing to its electron-deficient character, the degradation of PA in biosystems is difficult, making it an environmental contaminant.10 As an effect of the nitro and phenol functionalities, PA causes strong irritation to the skin and eyes and potential damage to organs of the respiratory system.8,9 Therefore rapid, cheap, sensitive and selective detection of picric acid is of prime importance. Various analytical techniques such as gas chromatography, ion mobility spectrometry, Raman spectroscopy, and fluorescence spectroscopy have been reported for the detection of NAC explosives.11 Furthermore, among the various techniques adopted for the detection of NACs, fluorescence signalling is superior with high sensitivity, specificity, real-time monitoring with a short response time and easy sample preparation,12 while other methods cannot be used in the field due to their high cost, and lack of selectivity and sensitivity.13 Analyte sensing by a fluorescent probe after binding with it is attributed to either enhancement or quenching of the fluorescence.14,15 Stoichiometric binding arising from collisional phenomena and less interference from fluctuation of the background fluorescence make a fluorescence enhancement method more preferable than quenching of the fluorescence for analyte sensing.16,17 Metal–organic frameworks18–22 and organic or inorganic conjugated polymers23,24 are mostly employed as fluorescent sensors for the detection of polynitroaromatics, particularly picric acid. In the field of NAC detection, the applied metal organic frameworks or quantum dots mostly contain cadmium and the pyrene class of sensors are limited by their toxicity.18,21,25,26 Among the available sensors for picric acid, detection by quenching of the fluorescence18,27–31 is more explored than ‘no quenching fluorescence sensors’.32–36 Currently p-terphenyl benzimidazolium-based molecular baskets,37 zinc–phthalocyanine framework based chemosensors with fluorescence quenching,38,39 electron-rich triphenylamine-based sensors with charge-transfer complex formation,40 electrogenerated thin films of a microporous polymer network responding to NACs,41 a glucopyranosyl-1,4-dihydropyridine chemosensor,42 a metal–organic framework [Cd3(TPT)2(DMF)2]·(H2O)0.5 for NAC detection,43 and a self-assembled nanoscopic organic cage for PA detection44 are some strategies focusing on NAC explosives detection. Most of the above discussed molecules or strategies are complicated so small molecules as fluorescence sensors are a better option as they are easy to synthesize and their properties can be easily tuned by structural modifications for the desired sensing specificity. BODIPY dyes are versatile fluorescent probes comprising various properties such as high chemical and photo-stabilities, high absorption coefficients and sharp fluorescence peaks with high quantum yields.45,46 With a number of possible structural modifications it is easy to design BODIPY luminophores with the desired spectroscopic and photophysical characteristics of a chemosensor.45–50 Some sensors with aromatic amines showed selectivity and sensitivity for picric acid but they are synthetically challenging.22,51 Considering the entire dilemma we have presented the current BODIPY probe as a selective turn on fluorescent chemosensor for picric acid detection among other NACs. This molecule possesses very weak fluorescence which gets enhanced as a consequence of the inhibition of the photoinduced electron transfer (PET) phenomenon. A perpendicular meso diaminophenyl moiety quenches the emission from the BODIPY core through PET utilising its lone pairs which are now associated with PA by a static interaction, leaving the BODIPY core to fluoresce, demonstrating the fluorescence turn on PA sensing.Experimental
Fluorescence spectra were recorded on a Varian Cary Eclipse Spectrofluorometer. UV/visible spectra were recorded on a Perkin Elmer Lambda-25 spectrophotometer using a 10 mm path length quartz cell. The photophysical study was carried out in HPLC-grade solvents. NMR spectra were recorded in DMSO-d6 solution using Agilent-500 MHz instruments, and the chemical shifts are reported in δ values (ppm) relative to TMS. 3,5-Dinitrobenzoyl chloride was purchased from Sigma Aldrich. All solvents used for syntheses were of synthetic grade and purchased from s d Fine Chemicals, India. Other chemicals/reagents were purchased from commercial sources. The reactions were carried out under a nitrogen atmosphere and dichloromethane was dried by treatment with calcium hydride before use.Determination of apparent association constants (Ka) of PA binding with sensor
The binding constant was calculated from the emission intensity–titration plot of 1/(I − I0) as a function of 1/[PA] in moles. I0 and I are the fluorescence intensities of BODIPY-2 without and with added PA. BODIPY-2 with a concentration of 1 × 10−6 mol L−1 in absolute ethanol was used for the fluorescence titration studies with PA. The PA concentration was varied from 4 to 13 eq. for this titration. The apparent binding constant for the formation of the complex was evaluated using a Benesi–Hildebrand plot.Estimation of detection limit
Fluorescence titration of the probe (conc. = 1 × 10−6 mol L−1) with PA in ethanol was carried out by adding aliquots of PA from 1 to 12 equivalents. The standard deviation (SD) was obtained from 10 blank readings of the probe (conc. = 1 × 10−6 mol L−1). From the plot of the fluorescence intensity as a function of the added PA eq., the slope was obtained. Then the detection limit (DL) was calculated from the equation DL = (3 × SD)/slope.Determination of the binding stoichiometry
Stoichiometry of the probe–analyte was determined using a continuous variation method known as a Job’s plot. In this experiment the total concentration of the probe and analyte ([BODIPY] + [PA]) was kept constant (30 μM) and the ratio was varied for 10 different fractions. The Job’s plot was obtained by plotting the fluorescence intensity (I − I0) as a function of the mole fraction of PA.Computational method
All DFT computations were performed using the Gaussian 09 (G09) program.52,53 Full geometry optimization of BODIPY-2 and its complex with PA were carried out using the hybrid functional B3LYP and the Pople’s double zeta basis set 6-31G(d).54,55Synthesis of BODIPY-1 and 2
2,4 Dimethyl pyrrole was prepared using the reported method.56 BODIPY-1 and BODIPY-2 were synthesized with small modifications to the reported procedure (Scheme 1).57 BODIPY-1 & 2 were characterised using 1H and 13C NMR.BODIPY-1: 1H NMR (500 MHz, CDCl3) δ (ppm) 9.19 (t, J = 2.1 Hz, 1H), 8.59 (d, J = 2.1 Hz, 2H), 6.07 (s, 2H), 2.59 (s, 6H), 1.37 (s, 6H).
BODIPY-1: 13C NMR (126 MHz, CDCl3) δ (ppm) 158.00, 149.01, 141.78, 138.81, 134.50, 130.67, 129.13, 122.59, 119.34, 15.38, 14.75.
BODIPY-2: 1H NMR (500 MHz, DMSO-d6) δ (ppm) 6.13 (s, 2H), 5.90 (t, J = 2 Hz, 1H), 5.67 (d, J = 2.0 Hz, 2H), 4.95 (s, 4H), 2.41 (s, 6H), 1.68 (s, 6H).
BODIPY-2: 13C NMR (126 MHz, DMSO-d6) δ (ppm) 154.41, 150.68, 144.70, 143.23, 135.11, 130.92, 121.20, 101.48, 100.21, 14.63, 14.20.
Results and discussion
For the photophysical study, absolute ethanol was the most suitable solvent among others. BODIPY-2 shows a strong absorption peak in absolute ethanol at 499 nm and very weak emission at 513 nm. The weak emission is attributed to efficient fluorescence quenching resulting from the photoinduced electron transfer phenomenon. BODIPY, having methyl groups at the 1 and 7 positions, keeps the meso phenyl ring out of the plane of the BODIPY core (Fig. 7a) and thus there is no conjugation between them, but when this meso phenyl ring is substituted with amino groups it impedes the emission. The amino groups on the angled meso phenyl ring contribute to the photoinduced electron transfer which suppresses the emission of the molecule. The engagement of the electron density on the amino groups with other groups could reset the emission which is the idea behind the construction of the sensor. Two amino groups were placed on the meso phenyl ring meta to each other with the expectation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
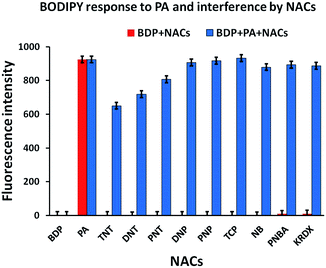
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry with PA for better values of the detection limit and binding constant. We screened different nitroaromatics (10 eq. each) i.e. picric acid (PA), p-nitrotoluene (PNT), 2,4-dinitrotoluene (DNT), 2,4,6-trinitrotoluene (TNT), p-nitrobenzoic acid (PNBA), p-nitrophenol (PNP), 2,4-dinitrophenol (DNP), 2,4,6-trichlorophenol (TCP), nitrobenzene (NBz) and ketoRDX (kRDX) with a 5 μM solution of the BODIPY probe which shows a high selectivity towards PA among the others with a 300 fold enhancement of the fluorescence intensity and a quick response (with just gentle shaking in <10 seconds) in absolute ethanol. Fig. 1 shows the graph for the recognition of the different NACs with the BODIPY probe. The specificity of the probe towards PA comes from the efficiency of the association between them. Though the electron withdrawing effect of the nitro groups plays a role in the interactions, the other NACs were insensitive to the probe.
2 binding stoichiometry with PA for better values of the detection limit and binding constant. We screened different nitroaromatics (10 eq. each) i.e. picric acid (PA), p-nitrotoluene (PNT), 2,4-dinitrotoluene (DNT), 2,4,6-trinitrotoluene (TNT), p-nitrobenzoic acid (PNBA), p-nitrophenol (PNP), 2,4-dinitrophenol (DNP), 2,4,6-trichlorophenol (TCP), nitrobenzene (NBz) and ketoRDX (kRDX) with a 5 μM solution of the BODIPY probe which shows a high selectivity towards PA among the others with a 300 fold enhancement of the fluorescence intensity and a quick response (with just gentle shaking in <10 seconds) in absolute ethanol. Fig. 1 shows the graph for the recognition of the different NACs with the BODIPY probe. The specificity of the probe towards PA comes from the efficiency of the association between them. Though the electron withdrawing effect of the nitro groups plays a role in the interactions, the other NACs were insensitive to the probe.
 | ||
| Fig. 1 Fluorescence responses of BODIPY-2 (5 μM, λex = 499 nm) to the addition of various NACs (10 equiv.) inset: magnified view of the recognition of different NACs. | ||
The high selectivity of the BODIPY probe to PA has been attributed to its specific binding with the analyte because of the proton transfer from PA to the amino group in the sensor which is in accordance with the static nature of the association. This selectivity is further supported on the basis of the pKa values of the NACs. PA has a pKa value of 0.38 which is far less than its structurally similar phenolic and nitroaromatic competitors such as DNP (4.11), PNP (7.15), PNBA (3.41), TCP (6.23) and TNT (11.99). Thus, as the most acidic, PA is preferred over all the screened NACs. Excluding the large enhancement in the fluorescence intensity of the BODIPY sensor with PA, other signals remained almost unaltered indicating that it has the highest specificity towards PA. The interference of other NACs for PA sensing by the sensor was studied by adding different NACs (10 eq. each) into a 5 μM solution of BODIPY + 10 eq. PA in absolute ethanol. This competitive binding experiment showed that there was no considerable lowering in the fluorescence intensity upon adding the NACs except for PNT, DNT and TNT for which an average 20% fall was observed. Fig. 2 shows the histogram of the recognition and interference study. The binding constant derived from the fluorescence titration data in accordance with the Benesi–Hildebrand plot was found to be Ka = 1.91 × 104 M−1 (Fig. 3).
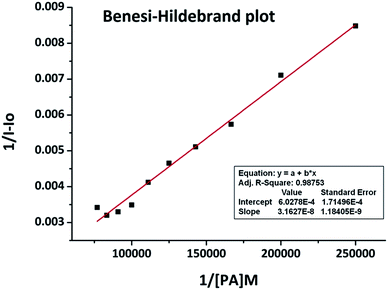 | ||
| Fig. 3 Benesi–Hildebrand plot of BODIPY-2 with PA in absolute ethanol at λex = 499 nm. The binding constant (Ka) = 1.91× 104 M−1. | ||
The sensitivity of the BODIPY probe towards PA was counted on the scale of the detection limit. From the changes in the PA concentration dependent fluorescence intensity, the detection limit was estimated to be 0.65 ppb. For the Benesi–Hildebrand plot and detection limit calculation, fluorometric titrations of BODIPY-2 with 1–12 equiv. PA were performed (Fig. S1, ESI†). The Job’s plot confirmed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the complexation between the host and guest. The fluorescence intensity changes more rapidly when the concentration of PA increased from 0.5 to 1 equiv., implying a 1
1 stoichiometry of the complexation between the host and guest. The fluorescence intensity changes more rapidly when the concentration of PA increased from 0.5 to 1 equiv., implying a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation between BODIPY-2 and PA (Fig. 4).
1 complexation between BODIPY-2 and PA (Fig. 4).
 | ||
| Fig. 4 Job’s plot of the BODIPY probe with PA according to the results of the continuous variation method at λex = 499 nm in absolute ethanol [BODIPY-2] + [PA] = 30 μM. | ||
A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry was expected between the probe and PA for the larger value of the binding constant as BODIPY has two amino groups on the meso phenyl ring which can associate with two molecules of PA independently, but it was 1
2 binding stoichiometry was expected between the probe and PA for the larger value of the binding constant as BODIPY has two amino groups on the meso phenyl ring which can associate with two molecules of PA independently, but it was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. To have more understanding of the binding stoichiometry we carried out a 1H NMR titration experiment. The 1H NMR spectra of BODIPY-2 in DMSO-d6 were recorded with 1, 2 and 4 equivalents of PA added independently. Fig. 5 shows the stacked graph of these titration experiments. After addition of 1 equivalent of PA to BODIPY-2 (1
1. To have more understanding of the binding stoichiometry we carried out a 1H NMR titration experiment. The 1H NMR spectra of BODIPY-2 in DMSO-d6 were recorded with 1, 2 and 4 equivalents of PA added independently. Fig. 5 shows the stacked graph of these titration experiments. After addition of 1 equivalent of PA to BODIPY-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry), peak c (for the two symmetric protons on the meso phenyl ring) and peak e (for the 2 and 6 positioned protons on the BODIPY core) shifted to the deshielded region by 0.68 ppm and 0.30 ppm, respectively. Another peak, d (for the proton between the two amino groups on the phenyl ring), comparatively was more shifted towards the deshielded region by 0.74 ppm as the electron density from the neighbouring amino group was engaged with the added PA. The peak f (at 4.95 ppm for the amino protons) in the plain BODIPY spectrum vanished with the addition of PA. Thus the aromatic region NMR signals were altered efficiently with the addition of PA. When the NMR spectrum was recorded with 2 equivalents of added PA (2
1 stoichiometry), peak c (for the two symmetric protons on the meso phenyl ring) and peak e (for the 2 and 6 positioned protons on the BODIPY core) shifted to the deshielded region by 0.68 ppm and 0.30 ppm, respectively. Another peak, d (for the proton between the two amino groups on the phenyl ring), comparatively was more shifted towards the deshielded region by 0.74 ppm as the electron density from the neighbouring amino group was engaged with the added PA. The peak f (at 4.95 ppm for the amino protons) in the plain BODIPY spectrum vanished with the addition of PA. Thus the aromatic region NMR signals were altered efficiently with the addition of PA. When the NMR spectrum was recorded with 2 equivalents of added PA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry) the signals already altered by 1 equivalent of PA were not shifted further considerably and the same was also found for 4 added equivalents. This observation reveals that there was the possibility of only 1
1 stoichiometry) the signals already altered by 1 equivalent of PA were not shifted further considerably and the same was also found for 4 added equivalents. This observation reveals that there was the possibility of only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry (Fig. 6).
1 binding stoichiometry (Fig. 6).
 | ||
| Fig. 5 The stacked 1H NMR titration spectra (500 MHz) of the BODIPY probe with 0, 1, 2 and 4 equivalents of PA in DMSO-d6. | ||
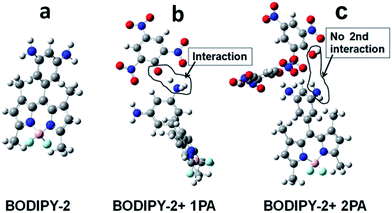
To get further insight into the binding nature, DFT computation was carried out for BODIPY-2, BODIPY-2 + 1 eq. PA and BODIPY-2 + 2 eq. PA. Three structures were optimised at B3LYP/6-31g(d) level using the Gaussian-09 software programme. Fig. 7 shows the optimised views of these structures. Fig. 7b shows the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding in which one of the amino groups on the meso phenyl ring of BODIPY-2 is protonated from PA while the picrate anion is stabilised by delocalisation of the charge over the trinitro-substituted ring. Fig. 7c shows again 1
1 binding in which one of the amino groups on the meso phenyl ring of BODIPY-2 is protonated from PA while the picrate anion is stabilised by delocalisation of the charge over the trinitro-substituted ring. Fig. 7c shows again 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding while the second PA molecule becomes dissociated by virtue of the molecular geometry.
1 binding while the second PA molecule becomes dissociated by virtue of the molecular geometry.
In the light of the Job’s plot, 1H NMR titration and DFT optimisation study, it is confirmed that only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding is possible between PA and the BODIPY probe. The reason behind this might be explained by the fact that after protonation of one of the amino groups with PA, the meso diamine phenyl ring becomes electron deficient. This makes it difficult for further association between the remaining amino group and the second PA molecule because of the molecular geometry constraint revealed by the DFT optimisation. Isodensity plots of these optimised structures at B3LYP/6-31g(d) level show that the LUMO level of BODIPY-2 has some charge density on the meso diamine phenyl ring which contributes to the PET mechanism (Fig. S3, ESI†). In the case of both the 1 and 2 equiv. PA associated structures, there was no charge density on the meso diaminophenyl ring at the LUMO level which erases any possibility of assistance for the PET process from the meso position of BODIPY. Thus when one NH2 group associates with a PA molecule it quaternizes (+NH3) and makes the meso phenyl ring electron deficient so that the lone pair of the other NH2 group is delocalised on the phenyl ring more efficiently than in the case of unassociated BODIPY-2. Further electron density from the remaining free NH2 and the phenyl ring is pulled by the electron deficient complex of +NH3 and the picrate anion. These things leave the still free NH2 group with much less electron density compared to that of the unassociated BODIPY-2 and hence it neither binds with another PA molecule nor contributes to the PET to limit the binding stoichiometry to 1
1 binding is possible between PA and the BODIPY probe. The reason behind this might be explained by the fact that after protonation of one of the amino groups with PA, the meso diamine phenyl ring becomes electron deficient. This makes it difficult for further association between the remaining amino group and the second PA molecule because of the molecular geometry constraint revealed by the DFT optimisation. Isodensity plots of these optimised structures at B3LYP/6-31g(d) level show that the LUMO level of BODIPY-2 has some charge density on the meso diamine phenyl ring which contributes to the PET mechanism (Fig. S3, ESI†). In the case of both the 1 and 2 equiv. PA associated structures, there was no charge density on the meso diaminophenyl ring at the LUMO level which erases any possibility of assistance for the PET process from the meso position of BODIPY. Thus when one NH2 group associates with a PA molecule it quaternizes (+NH3) and makes the meso phenyl ring electron deficient so that the lone pair of the other NH2 group is delocalised on the phenyl ring more efficiently than in the case of unassociated BODIPY-2. Further electron density from the remaining free NH2 and the phenyl ring is pulled by the electron deficient complex of +NH3 and the picrate anion. These things leave the still free NH2 group with much less electron density compared to that of the unassociated BODIPY-2 and hence it neither binds with another PA molecule nor contributes to the PET to limit the binding stoichiometry to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. On the basis of the acidic proton transfer from PA to the probe, and the DFT and NMR studies we conclude that the association is of an electrostatic nature, which is further supported by a salt screening effect study. The salt screening effect is a tool for determining the electrostatic interaction between sensor and analyte.58
1. On the basis of the acidic proton transfer from PA to the probe, and the DFT and NMR studies we conclude that the association is of an electrostatic nature, which is further supported by a salt screening effect study. The salt screening effect is a tool for determining the electrostatic interaction between sensor and analyte.58
To study this effect, NaNO3 salt with a concentration (0.1 M) more than PA (10 μM) was added to a solution of the BODIPY-2 probe (5 μM) and PA (Fig. 8). The result shows that after addition of the NaNO3 salt, the already enhanced fluorescence from the sensor + PA mixture gets diminished to almost the original intensity of the unbound sensor as the added salt, with a higher concentration than PA, eliminates the PA from the vicinity of the binding sites. This demonstrates the electrostatic association and proves why the probe is so sensitive and selective to PA over other NACs.
 | ||
| Fig. 8 Salt screening effect study by the interference of added NaNO3 salt to PA sensing with the BODIPY probe. | ||
Conclusion
We presented a simple meso diaminophenyl BODIPY probe for picric acid detection up to the ppb level. This probe is highly selective towards picric acid and non-sensitive to other equivalent NACs which have minimal interference while sensing. Masking of the PET process by picric acid through its association with the parent non-fluorescent BODIPY probe is effectively utilised to enhance the fluorescence signal. The binding nature is explained with different plots and supported by 1H NMR and computational study. Thus the less highlighted BODIPY probe and PET process in the field of picric acid sensing have been investigated which could inspire further innovation in the area.Acknowledgements
One of the authors (Yogesh Erande) is thankful to University Grant Commission-SAP, India for financial support by way of Junior and Senior Research Fellowships.References
- S. Singh, J. Hazard. Mater., 2007, 144, 15–28 CrossRef CAS PubMed.
- P. Cooper, Explosive Engineering, Wiley-VCH, 1996, p. 33 Search PubMed.
- J. Akhavan, R. Soc. Chem., 2004, 2nd edn.
- John Wiley Sons New York, 2000, IIB, 980.
- E. H. Volwiler, Ind. Eng. Chem., 1926, 18, 1336–1337 CrossRef CAS.
- D. T. Meredith and C. O. Lee, J. Am. Pharm. Assoc., 1939, 28, 369 CrossRef CAS PubMed.
- C. Beyer, U. Böhme, C. Pietzsch and G. Roewer, J. Organomet. Chem., 2002, 654, 187–201 CrossRef CAS.
- V. Pimienta, R. Etchenique and T. Buhse, J. Phys. Chem. A, 2001, 105, 10037–10044 CrossRef CAS.
- in Resource of National Institutes of Health.
- J. Shen, J. Zhang, Y. Zuo, L. Wang, X. Sun, J. Li, W. Han and R. He, J. Hazard. Mater., 2009, 163, 1199–1206 CrossRef CAS PubMed.
- Y. Salinas, R. Martínez-Máñez, M. D. Marcos, F. Sancenón, A. M. Costero, M. Parra and S. Gil, Chem. Soc. Rev., 2012, 41, 1261–1296 RSC.
- M. E. Germain and M. J. Knapp, Chem. Soc. Rev., 2009, 38, 2543–2555 RSC.
- E. Wallis, T. M. Griffin, N. Popkie Jr, M. A. Eagan and R. F. McAtee, Proc. SPIE, 2005, 54, 5795 Search PubMed.
- T. Q. Duong and J. S. Kim, Chem. Rev., 2010, 110, 6280–6301 CrossRef PubMed.
- R. Martínez-Máñez and F. Sancenón, Chem. Rev., 2003, 103, 4419–4476 CrossRef PubMed.
- S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871 RSC.
- J.-S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864–11873 CrossRef CAS.
- S. S. Nagarkar, B. Joarder, A. K. Chaudhari, S. Mukherjee and S. K. Ghosh, Angew. Chem., Int. Ed., 2013, 52, 2881–2885 CrossRef CAS PubMed.
- Y. Wang and Y. Ni, Anal. Chem., 2014, 86, 7463–7470 CrossRef CAS PubMed.
- V. Béreau, C. Duhayon and J.-P. Sutter, Chem. Commun., 2014, 50, 12061–12064 RSC.
- B. Liu, C. Tong, L. Feng, C. Wang, Y. He and C. Lü, Chemistry, 2014, 20, 2132–2137 CrossRef CAS PubMed.
- N. Venkatramaiah, D. M. G. C. Rocha, P. Srikanth, F. A. Almeida Paz and J. P. C. Tomé, J. Mater. Chem. C, 2015, 3, 1056–1067 RSC.
- A. Narayanan, O. P. Varnavski, T. M. Swager and T. Goodson, J. Phys. Chem. C, 2008, 112, 881–884 CAS.
- X.-G. Li, Y. Liao, M.-R. Huang, V. Strong and R. B. Kaner, Chem. Sci., 2013, 4, 1970 RSC.
- M. S. Meaney and V. L. McGuffin, Anal. Chim. Acta, 2008, 610, 57–67 CrossRef CAS PubMed.
- K. Zhang, H. Zhou, Q. Mei, S. Wang, G. Guan, R. Liu, J. Zhang and Z. Zhang, J. Am. Chem. Soc., 2011, 133, 8424–8427 CrossRef CAS PubMed.
- J.-D. Xiao, L.-G. Qiu, F. Ke, Y.-P. Yuan, G.-S. Xu, Y.-M. Wang and X. Jiang, J. Mater. Chem. A, 2013, 1, 8745 CAS.
- B. Roy, A. K. Bar, B. Gole and P. S. Mukherjee, J. Org. Chem., 2013, 78, 1306–1310 CrossRef CAS PubMed.
- S. Kumar, N. Venkatramaiah and S. Patil, J. Phys. Chem. C, 2013, 117, 7236–7245 CAS.
- P. Vishnoi, S. Sen, G. N. Patwari and R. Murugavel, New J. Chem., 2015, 39, 886–892 RSC.
- K. D. Prasad and T. N. G. Row, RSC Adv., 2014, 4, 45306–45310 RSC.
- G. Sivaraman, B. Vidya and D. Chellappa, RSC Adv., 2014, 4, 30828 RSC.
- A. Yadav and R. Boomishankar, RSC Adv., 2015, 5, 3903–3907 RSC.
- R. Chopra, V. Bhalla, M. Kumar and S. Kaur, RSC Adv., 2015, 5, 24336–24341 RSC.
- W. C. T. Jason and C. Sancheza, J. Mater. Chem., 2008, 18, 3143–3156 RSC.
- S. Sun, Y. P. Y. Xu, B. Li, W. Li and J. Zhao, Chem. Commun., 2013, 49, 4764–4766 RSC.
- S. Sandhu, R. Kumar, P. Singh, A. Mahajan, M. Kaur and S. Kumar, ACS Appl. Mater. Interfaces, 2015, 7, 10491–10500 CAS.
- A. Gupta, Y.-A. Kang, M.-S. Choi and J. S. Park, Sens. Actuators, B, 2015, 209, 225–229 CrossRef CAS PubMed.
- N. Venkatramaiah, D. M. G. C. Rocha, P. Srikanth, F. A. Almeida Paz and J. P. C. Tomé, J. Mater. Chem. C, 2015, 3, 1056–1067 RSC.
- A. Chowdhury and P. S. Mukherjee, J. Org. Chem., 2015, 80, 4064–4075 CrossRef CAS PubMed.
- A. U. Palma-Cando and U. Scherf, ACS Appl. Mater. Interfaces, 2015, 150506112911002 Search PubMed.
- O. Pinrat, K. Boonkitpatarakul, W. Paisuwan, M. Sukwattanasinitt and A. Ajavakom, Analyst, 2015, 140, 1886–1893 RSC.
- C. Zhang, L. Sun, Y. Yan, J. Li, X. Song, Y. Liu and Z. Liang, Dalton Trans., 2015, 44, 230–236 RSC.
- K. Acharyya and P. S. Mukherjee, Chem. Commun., 2014, 50, 15788–15791 RSC.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed.
- O. Buyukcakir, O. A. Bozdemir, S. Kolemen, S. Erbas and E. U. Akkaya, Org. Lett., 2009, 11, 4644–4647 CrossRef CAS PubMed.
- J.-H. Olivier, A. Haefele, P. Retailleau and R. Ziessel, Org. Lett., 2010, 12, 408–411 CrossRef CAS PubMed.
- H. He, P.-C. Lo, S.-L. Yeung, W.-P. Fong and D. K. P. Ng, Chem. Commun., 2011, 47, 4748–4750 RSC.
- A. B. Nepomnyashchii, S. Cho, P. J. Rossky and A. J. Bard, J. Am. Chem. Soc., 2010, 132, 17550–17559 CrossRef CAS PubMed.
- A. Ding, L. Yang, Y. Zhang, G. Zhang, L. Kong, X. Zhang, Y. Tian, X. Tao and J. Yang, Chemistry, 2014, 20, 12215–12222 CrossRef CAS PubMed.
- M. Robb, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, B. J. Nakatsuji, H. Caricato, M. Li, X. Hratchian, H. P. Izmaylov, A. F. F. R. Zheng, G. Sonnenberg, J. L. Hada, M. Ehara, K. Toyota, F. D. Hasegaw and C. J. Hasegaw, Gaussian 09 revision C01, 2010 Search PubMed.
- O. Treutler and R. Ahlrichs, J. Chem. Phys., 1995, 102, 346 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- E. V. Antina, G. B. Guseva, A. E. Loginova, A. S. Semeikin and A. I. V’yugin, Russ. J. Gen. Chem., 2010, 80, 2374–2381 CrossRef CAS.
- Q. Li, J. Xu, Y. Yue, Y. Liao and S. Shao, Anal. Methods, 2014, 6, 6531 RSC.
- G. He, H. Peng, T. Liu, M. Yang, Y. Zhang and Y. Fang, J. Mater. Chem., 2009, 19, 7347 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17932g |
| This journal is © The Royal Society of Chemistry 2015 |