Conjugation of polyethylenimine and its derivatives to carbon-encapsulated iron nanoparticles†
Artur Kasprzak*a,
Magdalena Popławskaa,
Michał Bystrzejewskib,
Olga Łabędźb and
Ireneusz P. Grudzińskic
aFaculty of Chemistry, Warsaw University of Technology, 00-664 Warsaw, Poland. E-mail: akasprzak@beryl.ch.pw.edu.pl
bFaculty of Chemistry, University of Warsaw, 02-093 Warsaw, Poland
cFaculty of Pharmacy, Medical University of Warsaw, 02-097 Warsaw, Poland
First published on 28th September 2015
Abstract
Carbon-based nanomaterials functionalized by cationic polymers are interesting starting materials for the development of nanotheranostic systems. In this study, polyethylenimine (PEI) and its pre-synthesized derivatives were conjugated to carbon-encapsulated iron nanoparticles (CEINs). Branched PEIs of various molecular weight were derivatized. The aim of the polymer modification was to introduce carboxylic functionality to the PEI structure. Two different synthetic pathways were proposed: the amide-type reaction with succinic acid anhydride and reductive amination using p-formylbenzoic acid. The polyethylenimine derivatives were analyzed by means of spectroscopic methods (NMR and FT-IR). In order to determine the ratio of primary, secondary and tertiary amine groups in the modified polymers, inverted-gate 13C NMR spectroscopy was applied. Next, CEINs modified with two different surface carboxylic linkers were functionalized using pristine PEI and its derivatives. The conjugation of the polymer to the surface-modified nanoparticles was carried out using the carbodiimide–amine type reaction. The success of the conjugation process was confirmed by thermogravimetry and infrared spectroscopy. The morphological details were analyzed using transmission electron microscopy, whilst the surface zeta potential and the average particle size were determined by dynamic light scattering. It was found that the molecular weight of the polymer and the type of the surface linker were the key factors which crucially influenced the functionalization yield and the physicochemical features of the synthesized nanoplatforms. The best dispersion stability in aqueous media and the smallest mean hydrodynamic particle size was found for CEINs with the longer carboxylic linker.
1. Introduction
The treatment of serious diseases, such as cancer, in most cases is associated with aggressive and patient-unfriendly therapy. Most antineoplastic agents, especially those of alkylating compounds, antimetabolites, natural products of Vinca alkaloids, epipodophyllotoxins and some miscellaneous chemicals, widely used in modern anti-cancer therapies, have a very low or narrow therapeutic index.1–3 In preclinical studies of such drugs it means that a median lethal dose (LD50) of the drug is nearly the same as a median effective pharmacological dose (ED50) of this medicine. Chemotherapy of the tumor, if feasible, involves also a number of undesirable or adverse toxic events to patients. In other words, adverse reactions are a cost of modern anti-cancer therapy. Therefore, the anticipated benefit from any therapeutic decision must be balanced by the potential risk. Hence, there is an urgent need to develop and apply new methods of cancer treatment using nanomaterials possessing both early diagnostic and therapeutic features. An interesting and promising method is creating a multifunctional theranostic system, which allows personalization of anti-cancer therapy in humans.4–6The development of nanotechnology caused growing interest in this field of science in terms of the biomedical application of different nanomaterials. So far, many articles on the application of nanostructures in theranostic platforms have been published.7–12 Mura and Couvreur called those smart and versatile platforms ‘nanotheranostics’.13 Such nanostructures are constructed of four main building units including: (i) the nanostructure ‘core’, used as a nanocarrier and diagnostic contrast agent (most commonly for MR imaging), (ii) a drug carrier or a gene delivery non-biological vector, i.e. a cationic polymer, (iii) a targeting ligand, like a peptide, protein, aptamer or antibody, which recognizes and selectively binds into the molecular target (i.e. nuclear receptors in the tumor), and (iv) a therapeutic unit such as drugs, nucleic acids, proteins, and enzymes etc. In the first step of the nanotheranostic platform synthesis, most frequently, the nanomaterial–polymer conjugate is created. The concept of the building units is up to a further strategy to be used in anti-cancer therapy, but there are nanostructures and macromolecules that ceaselessly attract a great deal of attention, due to potential application in future human nanomedicine.
Carbon-based nanomaterials exhibit extraordinary features related to their size and physical properties.14–16 It was found that this group of materials is one of the best nanocarriers recently examined in preclinical anti-cancer studies and modern diagnostics. In particular, noteworthy are metal-cored carbon structures, which besides the above applications, constitute contrast-like agents, so they can be used, i.e. in magnetic resonance imaging (MRI). Undeniably, such magnetic nanomaterials are a very interesting proposal for building the units of the nanotheranostic ‘core’. For example, Bosi et al. showed that gadolinium (a strongly paramagnetic metal widely used in MR imaging) could be permanently locked into the C60 fullerene cage.17 Note that the toxicity of gadolinium in these kinds of structures, called ‘metallofullerenes’, is reduced in comparison to currently used positive contrast agents (i.e. Magnevist®). Carbon-encapsulated iron nanoparticles (CEINs), synthesized in our laboratory, exhibit even more interesting and promising shape- and size-dependant properties.18–20 These kinds of encapsulates (the core–shell type nanomaterial) are built of a spherical-shaped iron core and a tight carbon (graphene-like) coating of high chemical inertness. The carbon-encapsulation method is employed to retain the inherent magnetic properties of these nanomaterials, by protecting against adverse environmental factors and possible agglomeration and/or aggregation in different media including human fluids. Hence, CEINs platforms could be considered as a potential theranostic nanomaterial for addressing molecular diagnostics (mMRI) and therapy of cancers. Note that our previous research showed the cytotoxic effects of these magnetic nanoparticles on human and murine melanoma21 as well as lung carcinoma cells (in vitro).22
An interesting feature of carbon nanomaterials is the ability to adsorb or covalently bind macromolecules, i.e. polymers,23 biomolecules,24 and target drugs.25,26 The surface modification of nanomaterials (introducing functionalities) is being widely studied, due to the further possibility of covalent-type functionalization using various macromolecules. So far, many ways of introducing such functional groups (most commonly carboxylic functionalities) were proposed, for fullerenes,17 nanotubes,27,28 and graphene.29,30 Also, the introduction of such surface functionalities onto carbon-encapsulated iron nanoparticles was recently reported in our studies.31,32
After nanomaterial selection for the pre-nanotheranostic platform, a real challenge is the choice of the most appropriate and promising therapeutic-unit carrier. This kind of non-biological vector should provide adequate drug/gene delivery and stabilization of the transferred therapeutic medicine under in vivo conditions.
Cationic polymers, i.e. chitosan and dextran, are widely studied due to their interesting features and therapeutic potential.33–35 It was found that this group of macromolecules showed encouraging abilities in the delivery of nucleic acids (i.e. siRNA),36,37 and drugs.38,39 Polyethylenimine (PEI) is one of the most interesting cationic polymers, which has all the required biomedical-field features and is widely used as the building unit in nanomaterial–polymer conjugates. To date, a variety of PEI–carbon material conjugates were proposed, i.e. using nanotubes,40–42 and graphene.43–45 The aim of the synthesis of such hybrids is to improve: (i) the solubility of the nanoplatform in aqueous/buffer media, (ii) the stability under physiological conditions and (iii) the therapeutic efficiency. It has to be noted that the cytotoxicity and toxicity of PEI is related to the molecular weight of this polymer: the cytotoxic potential (as well as toxicity) increases with the molecular weight. For example, Feng et al. showed that the cytotoxicity of PEI to HeLa cells (cervical cancer cell line) is associated with the molecular weight of the adsorbed polymer.46 For a PEI concentration of 300 mg L−1, the relative viability of HeLa cells for PEI 1.2 kDa was found to be approximately 90%, whilst only 20% was noted using PEI 10 kDa. Moreover, it was shown that creating such covalent or non-covalent carbon nanomaterial–PEI conjugates decreases the systemic toxicity of polyethylenimine. Establishing a proper balance between toxicity and pharmacological efficacy of the polymer used as a drug delivery system and/or gene transfer carrier is the main point and challenge in creating such nanotheranostics for modern anti-cancer therapy.
Since the first investigation into polyethylenimine’s application in the biomedical field, many publications on the surface chemical modification of this polymer have been reported. Some authors showed versatile and effective synthetic strategies of PEI derivatives, i.e. acylation,47,48 or introducing hydrophobic substituents.49,50 Also, more complex hybrid polymer structures were proposed, including chitosan–PEI copolymers,51 dextran–PEI polycationic vectors,52 and pullulan–PEI–folic acid conjugates.53 Most commonly, the biocompatibility of polyethylenimine is improved, by grafting PEI with poly(ethylene glycol) (PEG).54,55 These results suggest the very important fact that PEI modifications result in the reduction of the polymer toxicity, as well as an increase of the drug delivery or gene transfection efficiency in biological systems. However, both of these features are heavily dependent on the molecular weight of the used polyethylenimine. Undeniably also, PEI derivatives decorated with specific functionalities (i.e. carboxylic or sulfhydryl groups) allow for further conjugation-type reactions. In terms of the nanotheranostic synthesis these functionalities constitute incredibly important starting points (chemical targets) for structural expansion of the pre-theranostic wireframe.
In this work, we have undertaken an attempt to synthesize the basic wireframe of the nanotheranostic system consisting of carbon-encapsulated iron nanoparticles and polyethylenimine. The biomedical potential of the PEI nanoconstruct prompted us to try answer the fundamental question on the possibility of the conjugation-type reaction between surface-modified CEINs with pristine PEI and pre-synthesized PEI derivatives.
2. Experimental section
2.1 Materials and instrumentation
Carbon-encapsulated iron nanoparticles were synthesized using the so-called carbon arc route. The nanoparticle synthesis strategy and linker surface-modification were described elsewhere.31 The arc plasma synthesis yielded core–shell iron–carbon nanoparticles, which after purification are comprised of magnetic encapsulates with a diameter between 10 and 100 nm. The introduction of carboxylic functionalities onto the CEIN surface is based on two strategies: sonication of the pristine nanomaterial in a H2SO4/HNO3 mixture, or via a radical-type reaction with succinic acid acyl peroxide. Both modification strategies cause partial degradation of the carbon coating. Hereafter, the raw products are referred as Fe@C (pristine CEINs), Fe@C–COOH (shorter linker) and Fe@C–(CH2)2–COOH (longer linker). The carboxylic moiety content on the surface of the modified CEINs is 0.53 mmol g−1 and 1.42 mmol g−1 for Fe@C–COOH and Fe@C–(CH2)2–COOH, respectively.31Branched polyethylenimine of various molecular weights (PEI; Mw (by LS): 0.8 kDa, 25 kDa, and 750 kDa), succinic acid anhydride (SAA), p-formylbenzoic acid (pFBA), sodium borohydride, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCl), and N-hydroxysulfosuccinimide (NHS) were purchased from Sigma-Aldrich and were used as received without purification.
Thermogravimetric analysis (TGA) was performed with a TA Q-50 instrument under nitrogen atmosphere (heating rate: 5 °C min−1). The morphology was analyzed by transmission electron microscopy (TEM) using a Zeiss Libra Plus instrument (accelerating voltage: 120 kV). Dynamic light scattering (DLS) and the zeta potential measurements were performed using a Malvern Zetasizer instrument. The measurements were conducted on the nanomaterial samples suspended in distilled water (100 μg mL−1).
Fourier transformation infrared (FT-IR) spectra were recorded in transmission mode with a Thermo Scientific Nicolet iS5 spectrometer with a resolution of 8 cm−1. The samples were analyzed as pellets with dry KBr, whilst PEI and its derivatives were applied as a thin film onto a pellet made of pure KBr. 1H NMR and 13C NMR spectra were recorded on a Varian NMR System spectrometer (500 MHz and 125 MHz) or a Varian Gemini 2000 spectrometer (200 MHz and 50 MHz), in deuterium oxide. MestRe-C 2.0 software was used for NMR spectra simulation (MestRe-C NMR Data Processing Made Easy 4.9.9.6, 1996–2006, courtesy of F. J. Sardina, Universidad de Santiago de Compostela, Spain).
Sonication of the carbon materials was performed using a Bandelin Sonorex RK 100 H ultrasonic probe (ultrasonic peak output/HF power: 320 W/80 W; 35 kHz). The as-obtained suspensions were centrifuged on a MPW-260R centrifuge (5000 rpm, 24 °C).
The dialysis against water was carried out using 10k MCWO Snake-Skin® Dialysis Tubes (Thermo-Fisher Scientific). The lyophilisation was performed with FreeZone 1 liter Laboratory Lyophilizer (LABCONCO).
2.2 Synthesis of PEI–SA
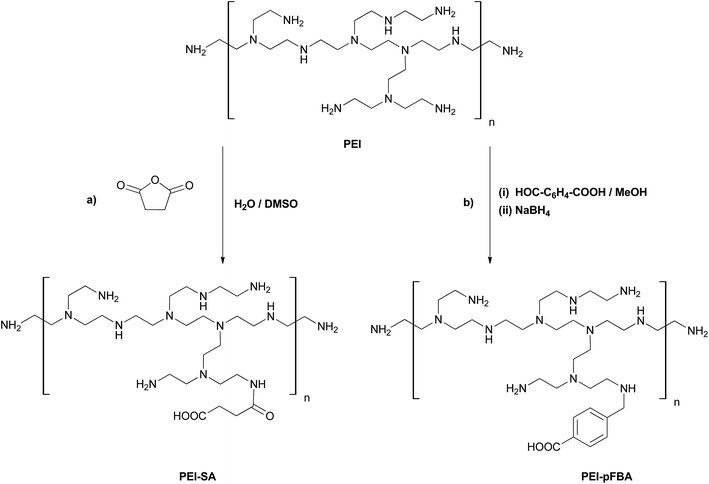
PEI–SA derivatives were synthesized by modifying the methods described elsewhere.54,56 The reaction scheme is shown in Fig. 1 (route a). First, the solution of 500 mg PEI (0.8 kDa, 25 kDa or 750 kDa) in 10 mL of distilled water was prepared. Then, 33 mg (0.33 mmol) of succinic acid anhydride was dissolved in 5 mL of DMSO and slowly added dropwise into the vigorously stirred PEI solution. The mixture was then stirred at room temperature for 24 h and then the obtained turbid-white mixture was dialyzed (10k MWCO membrane) against distilled water for 48 h. Finally, the product was lyophilized for 24 h. The average reaction yield was ca. 57%. Due to the membrane pore size, the PEI0.8k–SA derivative was not dialyzed, and was used in further research without purification.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O; amide I).
O; amide I).2.3 Synthesis of PEI–pFBA
PEI–pFBA derivatives were synthesized using the reductive amination approach. The reaction scheme is shown in Fig. 1 (route b). To the stirred solution of 500 mg PEI (0.8 kDa, 25 kDa or 750 kDa) in 10 mL of dried methanol, 37.5 mg (0.25 mmol) of p-formylbenzoic acid was added in one portion. The mixture was refluxed under argon atmosphere for 1 h and then stirred at room temperature for 24 h. Sequentially, 38.0 mg (1.0 mmol) of sodium borohydride was added. The reaction mixture was stirred for 4 h at room temperature and then the solvent was evaporated using a rotary evaporator. The as-obtained bright-yellow residue was dissolved in 5 mL of distilled water and extracted three times with 7 mL of ethyl acetate. The aqueous phase was dialyzed (10k MWCO membrane) against distilled water for 48 h. The obtained product was lyophilized for 24 h. The average reaction yield was ca. 50%. Once again, the PEI0.8k–pFBA derivative was not dialyzed, and was used in conjugation reactions with CEINs without further purification.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1600 (C
C), 1600 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).2.4 Conjugation of pristine PEI to surface-modified CEINs
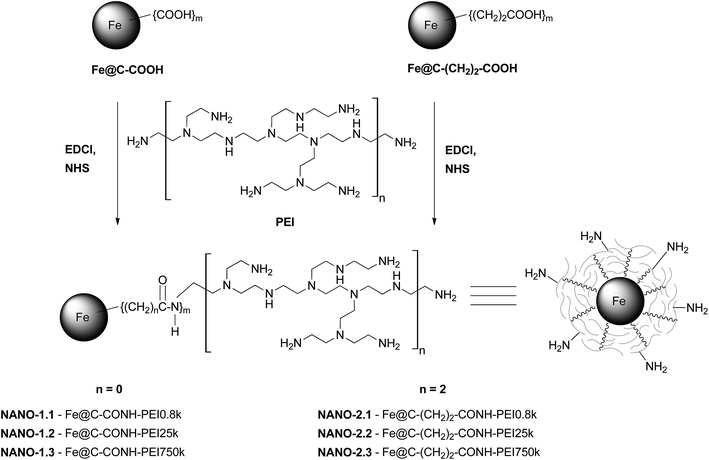
The reaction scheme is shown in Fig. 2. 20 mg of Fe@C–COOH or Fe@C–(CH2)2–COOH, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCl) (10 mol equivalent per 1 mol COOH groups) and N-hydroxysulfosuccinimide (NHS) (25 mol equivalent per 1 mol COOH groups) were sonicated in 10 mL of distilled water, for 1 h. Then, 200 mg of PEI (0.8 kDa, 25 kDa or 750 kDa) in 10 mL of distilled water was added to the carbon material suspension and sonicated for 5 h at room temperature. Afterwards, the obtained conjugate suspension was centrifuged several times (5000 rpm, 24 °C, 25 min) in methanol. The PEI content in the following supernatants was monitored by TLC (a ninhydrin test was used). Finally, the carbon material was dried at 45 °C for 24 h. The observed positive mass gain pre-indicated the success of the conjugation. Due to the highly hygroscopic features of this kind of nanomaterial–polymer conjugate, the observed mass gain could not be directly taken as the real conjugation yield.The obtained products were named as NANO-1.1–1.3 and NANO-2.1–2.3 for Fe@C–CONH–PEI and Fe@C–(CH2)2–CONH–PEI, respectively.
2.5 Conjugation of PEI–SA and PEI–pFBA to surface-modified CEINs
The reaction scheme is shown in Fig. 3. Prior to the conjugation 20 mg of Fe@C–COOH or Fe@C–(CH2)2–COOH, N-(3-dimethyl-aminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCl) (10 mol equivalent per 1 mol COOH groups) and N-hydroxysulfosuccinimide (NHS) (25 mol equivalent per 1 mol COOH groups) were sonicated in 10 mL of distilled water, for 3 h. Then, the supernatant was precisely separated from the nanoparticles and removed from the flask using a Pasteur pipette (carbon-encapsulated iron nanoparticles were immobilized using a magnet). Next, to the carbon material residue, a solution of 200 mg of the appropriate PEI derivative (PEI–SA or PEI–pFBA) in 10 mL of distilled water was added. The sonication was performed for 4 h at room temperature. The obtained suspension was centrifuged several times (5000 rpm, 24 °C, 30 min) with methanol, until the polymer was not present in a supernatant (a ninhydrin test was used). The final carbon material was dried at 45 °C for 24 h.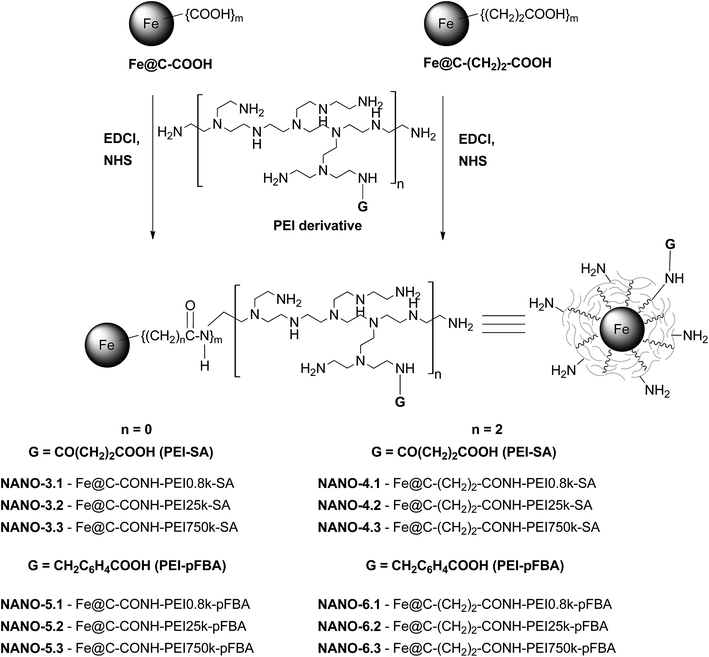 | ||
| Fig. 3 Conjugation of PEI derivatives (PEI–SA and PEI–pFBA) to carbon-encapsulated iron nanoparticles. | ||
The obtained products were named as NANO-3.1–3.3, NANO-4.1–4.3, NANO-5.1–5.3 and NANO-6.1–6.3 for Fe@C–CONH–PEI–SA, Fe@C–(CH2)2–CONH–PEI–SA, Fe@C–CONH–PEI–pFBA and Fe@C–(CH2)2–CONH–PEI–pFBA, respectively.
3. Results and discussion
3.1 Structural modification of PEI (synthesis of PEI–SA and PEI–pFBA)
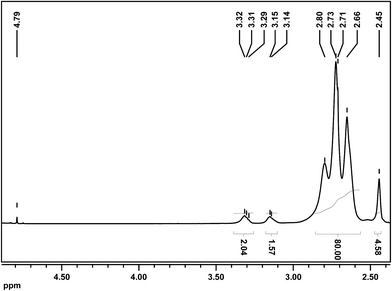
The analysis of the synthesized PEI derivatives was carried out using spectroscopic methods. First, 1H and 13C NMR spectra of the products were recorded. The 1H NMR spectra of succinated PEI 25 kDa (PEI25k–SA) and p-carboxybenzylated PEI 25 kDa (PEI25k–pFBA) are shown in Fig. 4 and 5, respectively (other 1H and 13C NMR spectra of the PEI derivatives are presented in Fig. S2, ESI†). There were no significant signal shifts of these derivatives in comparison with the pristine PEI (for 1H and 13C NMR spectra of pristine PEI, please see spectra 1–4 in Fig. S1 in the ESI†). The spectra of all the products consist of peaks corresponding to the protons of PEI 25 kDa and some new features that could be assigned to the introduced substituents. In the PEI25k–SA spectrum (Fig. 4) the signal from the methylene groups of succinic acid is present (2.45 ppm), but a more significant broad multiplet is located at 3.29–3.32 ppm. This signal corresponds to the methylene moiety at the primary amine group of PEI with the introduced succinic substituent ({PEI}–CH2–NH–CO–(CH2)2–COOH). Please note that the 2.45 ppm and 3.29–3.32 ppm signal ratio is about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and this finding confirms our supposition about peak assignment and the success of PEI modification. The characteristic signals of the aromatic ring protons are present (7.34–7.39 ppm and 7.85–7.93 ppm) for the PEI25k–pFBA sample (Fig. 5). The peak located at 3.79 ppm corresponds to the benzyl moiety ({PEI}–NH–CH2–C6H4–), whilst the broad multiplet at 3.25–3.30 ppm is assigned to the methylene moiety at the primary amine group of PEI with the introduced p-carboxybenzyl substituent ({PEI}–CH2–NH–CH2–C6H4–COOH). The peak ratio confirms the successful modification of PEI. Moreover, it is worth noting that peaks from the substrates (succinic acid anhydride or p-formylbenzoic acid) and their analogs (i.e. p-carboxybenzyl alcohol, due to the possible reduction of the substrate) are not observed.
1, and this finding confirms our supposition about peak assignment and the success of PEI modification. The characteristic signals of the aromatic ring protons are present (7.34–7.39 ppm and 7.85–7.93 ppm) for the PEI25k–pFBA sample (Fig. 5). The peak located at 3.79 ppm corresponds to the benzyl moiety ({PEI}–NH–CH2–C6H4–), whilst the broad multiplet at 3.25–3.30 ppm is assigned to the methylene moiety at the primary amine group of PEI with the introduced p-carboxybenzyl substituent ({PEI}–CH2–NH–CH2–C6H4–COOH). The peak ratio confirms the successful modification of PEI. Moreover, it is worth noting that peaks from the substrates (succinic acid anhydride or p-formylbenzoic acid) and their analogs (i.e. p-carboxybenzyl alcohol, due to the possible reduction of the substrate) are not observed.
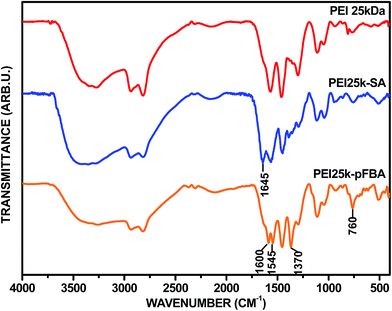
Fourier transformation infrared (FT-IR) spectroscopy was finally carried out to confirm the success of each modification. Fig. 6 shows the FT-IR spectra of pristine PEIs of various molecular weight. As can be seen, the spectra are very similar. The bands at the lowest wavelengths correspond to N–H stretching vibrations (3300–3450 cm−1) and aliphatic C–H stretching vibrations (2810–2930 cm−1). Incredibly important are two strong bands located at 1580 cm−1 and 1460 cm−1, which are associated with the N–H vibrations of 1° and 2° amino groups, respectively. The band at 1460 cm−1 also corresponds to the CH2 moiety. The peaks at 1300 cm−1 and in the range 1040–1120 cm−1 correspond to the C–N stretching vibrations. In Fig. 7 we present the spectra of two PEI derivatives: succinated PEI 25 kDa (PEI25k–SA) and p-carboxybenzylated PEI 25 kDa (PEI25k–pFBA) (data not shown for PEI 0.8 kDa and 750 kDa derivatives). The bands that are typical for pristine PEI appear in the spectrum of each derivative. For the PEI25k–SA derivative, the new absorption band at 1645 cm−1 can be clearly assigned to the imposition of the amide I band and the stretching C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration. In contrast, for the PEI25k–pFBA derivative, the new absorption band is located at 1600 cm−1. This feature can be attributed to the stretching vibrations of the C
O vibration. In contrast, for the PEI25k–pFBA derivative, the new absorption band is located at 1600 cm−1. This feature can be attributed to the stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety, as the result of the intramolecular interactions between the Ar–COOH substituent and the amino groups of PEI. Due to those electrostatic interactions the position of the considered absorption band is down-shifted in comparison to smaller molecules, e.g. benzoic acid. Moreover, for the PEI25k–pFBA derivative an evident intensity enhancement is seen for the bands located at 1370 cm−1 and 760 cm−1, which could be assigned to the C
O moiety, as the result of the intramolecular interactions between the Ar–COOH substituent and the amino groups of PEI. Due to those electrostatic interactions the position of the considered absorption band is down-shifted in comparison to smaller molecules, e.g. benzoic acid. Moreover, for the PEI25k–pFBA derivative an evident intensity enhancement is seen for the bands located at 1370 cm−1 and 760 cm−1, which could be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C skeletal vibration inside the aromatic ring and the C–H out-of-plane ring vibrations, respectively.
C skeletal vibration inside the aromatic ring and the C–H out-of-plane ring vibrations, respectively.
In order to evaluate the PEI modification degree, inverted-gate 13C NMR spectroscopy was applied. The signals in the spectra were assigned as was reported earlier.57,58 Thus, PEI 25 kDa and its derivatives were chosen as the representative polymer samples. We have found, that the ratio of primary, secondary and tertiary amine groups of pristine PEI 25 kDa (NH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH
NH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N) is 1
N) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12
1.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.89 (please see spectrum 1 in Fig. S3 in the ESI†), whereas, this ratio for the polymer derivatives was quite different and equalled 1
0.89 (please see spectrum 1 in Fig. S3 in the ESI†), whereas, this ratio for the polymer derivatives was quite different and equalled 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.62
1.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.32 and 1
1.32 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.69
1.69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.39, for PEI25k–SA and PEI25k–pFBA, respectively (please see spectra 2 and 3 in Fig. S3 in the ESI†). The ratio has changed quite significantly with the reduction in the number of primary amino groups in the synthesized polymer derivatives. Also, it could be calculated that the modification degree is about 10% (9.5% and 10.5% for PEI25k–SA and PEI25k–pFBA, respectively). Those observations prove that the modifications of PEI were successful and indicate the similar degree of modifications of the polymer using succinic acid anhydride and p-formylbenzoic acid.
1.39, for PEI25k–SA and PEI25k–pFBA, respectively (please see spectra 2 and 3 in Fig. S3 in the ESI†). The ratio has changed quite significantly with the reduction in the number of primary amino groups in the synthesized polymer derivatives. Also, it could be calculated that the modification degree is about 10% (9.5% and 10.5% for PEI25k–SA and PEI25k–pFBA, respectively). Those observations prove that the modifications of PEI were successful and indicate the similar degree of modifications of the polymer using succinic acid anhydride and p-formylbenzoic acid.
In most cases, the toxicity of PEI is explained by the high positive charge of PEI (and so a high zeta potential).47,59 Hence, the representative polymers PEI 25 kDa, PEI25k–SA and PEI25k–pFBA were sonicated in water (100 μg mL−1) and zeta potential (ZP) measurements were carried out. Firstly, PEI 25 kDa was dialyzed against water for 48 hours, then the obtained fractions (remaining in the dialysis tube and the water fraction after dialysis) were evaporated under reduced pressure and lyophilized. As expected, the zeta potential (ZP) of each fraction was found to be +23.2 mV, +29.0 mV and +19.0 mV for pristine PEI (without dialysis), PEI fraction >10 kDa and PEI fraction <10 kDa, respectively. Sequentially, the zeta potential of the PEI 25 kDa derivatives was measured, and was found to be +21.0 mV and +28.7 mV, for PEI25k–SA and PEI25k–pFBA, respectively. Please note that the zeta potential of the polymer derivatives should be compared with the ZP of PEI fraction >10 kDa, because the obtained products were dialyzed. Hence, the ZP of the PEI derivatives, in comparison to pristine PEI 25 kDa (fraction >10 kDa), is 1.31 and 1.01 fold lower for PEI25k–SA and PEI25k–pFBA, respectively. This finding shows that the succination and p-carboxybenzylation of PEI results in the reduction of the zeta potential of PEI (especially for the PEI–SA derivatives) due to the decreasing amount of available amino moieties in the polymer structure. If so, one has to consider the reduction of toxicity of PEI by synthesizing its carboxylic derivatives, combined with no significant decrease in the binding capacity of biomolecules.
3.2 Synthesis and characterization of PEI–CEIN conjugates
The synthesis of polymer conjugates was performed with the carbodiimide–amine type reaction (EDCl, NHS). First, the results of the conjugation of PEI and its derivatives to carbon-encapsulated iron nanoparticles were analyzed qualitatively. FT-IR spectroscopy is a convenient technique for the analysis of carbon materials and their conjugates with polymers. This technique in many cases allows determination of whether the conjugation reaction proceeded successfully. In Fig. 8 we present two representative FT-IR spectra of conjugates: (a) Fe@C–CONH–PEI (NANO-1.1–1.3) and (b) Fe@C–(CH2)2–CONH–PEI–pFBA (NANO-6.1–6.3) (for other FT-IR spectra, please see spectra 1–4 in Fig. S4 in the ESI†). For comparison, the spectra of the surface-functionalized CEINs are also shown. The bands that are characteristic for PEI appear in the spectrum of each conjugate, which means that the functionalization of CEINs was successful (please compare with the PEIs spectra in Fig. 6). Moreover, in contrast to the pristine Fe@C–(CH2)n–COOH (n = 0 or 2) spectra, the band located at 1710 cm−1, coming from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration in the carboxylic groups, is absent after the conjugation. It means that nearly all carboxylic functionalities on the surface of CEINs participated in the formation of covalent amide bonds (in other words the CEINs conjugated to PEIs do not contain free carboxylic groups). Also, the presence of PEI in the carbon materials can be confirmed by two weak bands found at the lowest wavelengths (please see Fig. S5 in the ESI†). Those features located at 2850 cm−1 and 2925 cm−1 can be assigned to C–H stretching vibrations in the CH2 moieties of PEI, which are clearly visible in the pristine PEIs spectra. Hence, it can be considered that all conjugation reactions were successful. Please note that due to the highly hygroscopic character of the obtained conjugates, the broad bands located in the spectrum beyond ca. 2950 cm−1 cannot be directly assigned to N–H stretching vibrations of PEI amine moieties and so this is regarded as the determinant of the success of the functionalization.
O vibration in the carboxylic groups, is absent after the conjugation. It means that nearly all carboxylic functionalities on the surface of CEINs participated in the formation of covalent amide bonds (in other words the CEINs conjugated to PEIs do not contain free carboxylic groups). Also, the presence of PEI in the carbon materials can be confirmed by two weak bands found at the lowest wavelengths (please see Fig. S5 in the ESI†). Those features located at 2850 cm−1 and 2925 cm−1 can be assigned to C–H stretching vibrations in the CH2 moieties of PEI, which are clearly visible in the pristine PEIs spectra. Hence, it can be considered that all conjugation reactions were successful. Please note that due to the highly hygroscopic character of the obtained conjugates, the broad bands located in the spectrum beyond ca. 2950 cm−1 cannot be directly assigned to N–H stretching vibrations of PEI amine moieties and so this is regarded as the determinant of the success of the functionalization.
 | ||
| Fig. 8 FT-IR spectra of PEI-CEIN conjugates: (a) NANO-1.1–1.3, and (b) NANO-6.1–6.3. See Fig. 2 and 3 for legends. | ||
To evaluate the amount of the polymer conjugated to Fe@C–COOH and Fe@C–(CH2)2–COOH, thermogravimetric analysis (TGA) was carried out. Firstly, the TGA curves of PEI of different molecular weights were analyzed (Fig. S6 in the ESI†). The first weight loss, between ca. 60–120 °C, is related to the presence of moisture. Further weight loss starts at ca. 210 °C and is accompanied by the total decomposition of the polymer (without formation of the char). As we reported earlier for the surface-modified CEINs, under inert atmosphere the weight loss in the range between 200 °C and 500 °C was found to be 3.5% and 5.1% for Fe@C–COOH and Fe@C–(CH2)2–COOH, respectively.31 Under nitrogen atmosphere, the magnetic core and the carbon coating do not undergo degradation (except in the range above 500 °C, due to trace amounts of oxygen resulting in the oxidation of the carbon phase). In Fig. 9 and 10, the thermogravimetric curves of two representative conjugate lines are presented: Fe@C–CONH–PEI (NANO-1.1–1.3; Fig. 9) and Fe@C–(CH2)2–CONH–PEI–pFBA (NANO-6.1–6.3; Fig. 10) (for other TGA curves, please see graphs 1–4 in Fig. S7 in the ESI†). A two-step decomposition is observed for the obtained conjugates. The first weight loss of ca. 2–3% in the temperature range between 60–120 °C is due to the presence of moisture. The further weight loss starts at ca. 200 °C and is completed at ca. 530 °C. The weight loss for each conjugate is incomparably greater than that for the pristine CEINs, which evidently indicates that the polymer was attached to surface-modified CEINs. Moreover, our studies showed that due to the covalent attachment of PEI onto CEINs, the polymer does not undergo total decomposition at ca. 375 °C, but at a higher temperature instead (compare representative DTG curves in Fig. S8 in the ESI†). This weight change is between 12% and 60%, but these numbers cannot be directly taken as the PEI content in the samples, due to the weight loss in this temperature range for pristine CEINs with a shorter or longer surface linker. Nevertheless, the PEI content (CPEI) in each conjugate can be calculated according to the equation: CPEI = (W − M − L) × 100%, where W is the observed weight loss for each conjugate, M is moisture, and L is the weight loss for the linker on the surface of appropriate CEINs. However, the L factor cannot be directly taken as the value for ‘PEI-free’ Fe@C–COOH and Fe@C–(CH2)2–COOH, due to the fact that carbon materials are covalently composed of the polymer and the nanoparticles. We have assumed that the L factor can be approximated as follows: L = LPnano × FCEINs, where LPnano is the appropriate value for pristine linker-modified CEINs (3.5% or 5.1%), and FCEINs (%) is the approximated relative fraction of CEINs in the carbon material, which was found to be between 50% and 88% (for the calculation method, please see Section IV in the ESI†). The CPEI (in other words, the conjugation yield) values are shown in Table 1. The PEI content was found to be between 6.5% and 52.4%. The highest conjugation yield is found for PEI 750 kDa and its derivatives, especially for Fe@C–CONH–PEI750k–SA (CPEI = 52.4%). The PEI content for CEINs conjugated with the pristine polymer is ca. 1.64–1.91 fold higher for CEINs with a longer surface linker, in comparison to the shorter one. It can be explained by the higher content and better accessibility of carboxylic moieties for the Fe@C–(CH2)2–COOH material. Moreover, the conjugation yield is larger for the polymer of a higher molecular weight, due to the increasing number of mer units in the polymer structure. Please note that for the conjugates containing pre-modified PEI, the relations described above are not always fulfilled, i.e. for NANO-3.3 (52.4%) and NANO-4.3 (21.2%), and for NANO-5.3 (15.0%) and NANO-6.3 (11%). Nevertheless, in most cases, using a high molecular weight PEI and CEINs with a longer surface linker implied a higher content of PEI in the obtained carbon material. Thus, two main determinants of the conjugation process can be distinguished: the accessibility and content of: (i) the primary amine groups of PEI and (ii) the surface linker on the surface of CEINs. For PEI–SA and PEI–pFBA, the number of first-order amine moieties depends on the polymer pre-modification yield and it may not be related only to the molecular weight of PEI. Importantly, possible intramolecular electrostatic interactions between pre-introduced carboxylic functionalities (PEI–SA and PEI–pFBA) and amine groups in the PEI structure can significantly influence the conjugation process. If so, one has to consider the electrostatic effects and the randomized, polymer-dependent process. For this kind of heterogeneous process, there is an appropriate set of parameters which is related to the accessibility of primary amine groups of PEI, PEI–SA, and PEI–pFBA and the convenient orientation (accessibility) of the surface-linker of CEINs and the reaction conditions.
 | ||
| Fig. 9 TGA curves (in nitrogen) of Fe@C–CONH–PEI (NANO-1.1–1.3). See Fig. 2 for legends. | ||
 | ||
| Fig. 10 TGA curves (in nitrogen) of Fe@C–(CH2)2–CONH–PEI–pFBA (NANO-6.1–6.3). See Fig. 3 for legends. | ||
| Structure | Content (%) | Structure | Content (%) |
|---|---|---|---|
| NANO-1.1 | 7.7 | NANO-2.1 | 14.6 |
| Fe@C–CONH–PEI0.8k | Fe@C–(CH2)2–CONH–PEI0.8k | ||
| NANO-1.2 | 6.5 | NANO-2.2 | 12.0 |
| Fe@C–CONH–PEI25k | Fe@C–(CH2)2–CONH–PEI25k | ||
| NANO-1.3 | 8.4 | NANO-2.3 | 13.8 |
| Fe@C–CONH–PEI750k | Fe@C–(CH2)2–CONH–PEI750k | ||
| NANO-3.1 | 7.0 | NANO-4.1 | 13.6 |
| Fe@C–CONH–PEI0.8k–SA | Fe@C–(CH2)2–CONH–PEI0.8k–SA | ||
| NANO-3.2 | 21.8 | NANO-4.2 | 17.7 |
| Fe@C–CONH–PEI25k–SA | Fe@C–(CH2)2–CONH–PEI25k–SA | ||
| NANO-3.3 | 52.4 | NANO-4.3 | 21.2 |
| Fe@C–CONH–PEI750k–SA | Fe@C–(CH2)2–CONH–PEI750k–SA | ||
| NANO-5.1 | 6.7 | NANO-6.1 | 8.8 |
| Fe@C–CONH–PEI0.8k–pFBA | Fe@C–(CH2)2–CONH–PEI0.8k–pFBA | ||
| NANO-5.2 | 6.8 | NANO-6.2 | 10.3 |
| Fe@C–CONH–PEI25k–pFBA | Fe@C–(CH2)2–CONH–PEI25k–pFBA | ||
| NANO-5.3 | 15.0 | NANO-6.3 | 11.0 |
| Fe@C–CONH–PEI750k–pFBA | Fe@C–(CH2)2–CONH–PEI750k–pFBA |
Transmission electron microscopy (TEM) studies were carried out to finally confirm the presence of PEI on the CEINs surface and to analyze the morphology of the conjugates. The representative microscopic images of the conjugates consisting of a shorter (a) and longer (b) surface linker are shown in Fig. 11. The surface functionalization of CEINs is clearly seen in comparison to the unmodified encapsulates (please compare with the TEM images of CEINs shown elsewhere21,31). The carbon encapsulate and its typical (graphitic) coating are surrounded by a shapeless and inhomogeneous PEI layer. Also, based on the TGA curves in oxygen (data not shown), the iron content in the conjugates was found to be similar to the starting value for CEINs with a shorter or longer surface linker. However those values are strictly connected with the polymer content in the conjugates.
 | ||
| Fig. 11 Representative TEM images of: (a) NANO-1.3 and (b) NANO-2.2. See Fig. 2 for legends. | ||
The samples of PEI25k–CEINs, as the representative conjugates, were suspended in water (100 μg mL−1) and subjected to dynamic light scattering (DLS) and zeta potential (ZP) measurements. The average particle size and surface zeta potential are listed in Table 2. The value of the zeta potential for 4 of the 5 samples is positive, due to the presence of a cationic-type polymer. Unexpectedly, the zeta potential of NANO-1.2 was found to be negative. This finding may be correlated with the lowest polymer content in NANO-1.2, and therefore a high CEINs fraction in this conjugate (pristine surface-modified CEINs exhibit a negative zeta potential). However, there is a need for further detailed study to explain this phenomenon. The ZP was found to be lower for the structures containing pristine PEI in comparison to conjugates with pre-modified PEI. The values of the ZP for NANO-3.2, NANO-4.2, NANO-5.2 and NANO 6.2 are higher than 30 mV and are quite similar (30–34.1 mV), which suggests good dispersion stability in water. The mean hydrodynamic particle size was found to be between 250 nm and 575 nm, and was 1.24–2.25 fold smaller for the conjugates comprising of the longer surface linker (that is, NANO-2.2, NANO-4.2 and NANO-6.2) in comparison to the shorter one. It is worth mentioning that the mean particle size (DLS measurements) for the pristine PEI 25 kDa solution in water (100 μg mL−1) was found to be 239 nm. The DLS and, especially, the zeta potential measurements suggest the dispersion stability in the experimental media. Interestingly, the best dispersion stability in aqueous media was found to be incredibly satisfactory for the samples containing longer surface linkers. The carbon material containing the longer linker, pre-sonicated in water (100 μg mL−1), remained in a stable dispersion form even for 60 days. This finding suggests that the measured zeta potential is not a main determinant of the stability of the obtained carbon material dispersion, but rather the length of the surface linker of CEINs. Please note that this feature of carbon materials (and other drug structures administered in the dispersion form) is very important for patient safety and an optimal therapeutic effect.
| Structure | Zeta potential (mV) | Size (nm) |
|---|---|---|
| a Carbon material samples were suspended in distilled water, with a concentration of 100 μg mL−1. | ||
| NANO-1.2 | −8.1 | 575 |
| Fe@C–CONH–PEI25k | ||
| NANO-2.2 | +18.1 | 256 |
| Fe@C–(CH2)2–CONH–PEI25k | ||
| NANO-3.2 | +32.2 | 479 |
| Fe@C–CONH–PEI25k–SA | ||
| NANO-4.2 | +32.2 | 383 |
| Fe@C–(CH2)2–CONH–PEI25k–SA | ||
| NANO-5.2 | +30.1 | 378 |
| Fe@C–CONH–PEI25k–pFBA | ||
| NANO-6.2 | +34.1 | 287 |
| Fe@C–(CH2)2–CONH–PEI25k–pFBA | ||
3.3 Study on the physical adsorption of PEI onto CEINs
The review of recent literature leads to the conclusion that there are two different pathways of creating pre-nanotheranostic platforms: covalent immobilization of macromolecules onto surface-modified nanomaterials or non-covalent modification (physical adsorption). The EDCl-mediated approach is the most encountered synthetic strategy of covalent attachment of high molecular weight molecules, i.e. proteins or cationic polymers, to surface-modified carbon nanomaterials.60 However, there is still a high probability of non-covalent electrostatic adsorption of a biomolecule. Hence, it is very important to verify the possibility of non-covalent adsorption for each newly synthesized nanomaterial–biomolecule conjugate. Therefore, we have checked the probability of the adsorption of PEI 0.8 kDa and 25 kDa onto CEINs. The polymer and carbon-encapsulated iron nanoparticles (pristine (without linker) and surface-modified (with the (CH2)2COOH linker)), were sonicated for 5 h. Then the suspensions were centrifuged several times in methanol and dried. The PEI content in the supernatants was monitored by TLC (a ninhydrin test was used). There was no weight gain in the Fe@C sample, whilst a weight gain of ca. 4–5% was observed in the Fe@C–(CH2)2–COOH samples. To confirm the supposition of PEI adsorption, thermogravimetric and spectroscopic (FT-IR) analyses were performed. There were no characteristic signals of PEI in the FT-IR spectra and any essential weight loss in the TGA curves for the linker-free nanomaterial samples was observed. For both the Fe@C–(CH2)2–COOH samples we have found characteristic features for surface-modified CEINs and PEI (please see Fig. S9 in the ESI†). The polymer content in each sample was ca. 10% wt according to TGA. Therefore, we can conclude that PEI adsorption onto linker-modified CEINs is very strong, whereas the polymer is not adsorbed onto unmodified CEINs.In order to reduce the PEI adsorption phenomena, while applying the EDCl–NHS-mediated approach, per 1 mol COOH groups on the surface of the carbon material, we have used 10 and 25 mol equivalent of EDCl and NHS, respectively. The carboxylic groups on the CEINs surface were activated before PEI addition. What is worth noticing is that in the non-covalently modified structures, a noticeable signal in the infrared spectra at ca. 1740 cm−1 was observed (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in the carboxylic groups; please see Fig. S9 in the ESI†), whilst this band was absent in the covalently synthesized structures. This means that all the carboxylic groups participate in the formation of covalent amide bonds while applying the EDCl–NHS-mediated synthetic pathway. Further research on the non-covalent PEI attachment is currently underway.
O stretching vibration in the carboxylic groups; please see Fig. S9 in the ESI†), whilst this band was absent in the covalently synthesized structures. This means that all the carboxylic groups participate in the formation of covalent amide bonds while applying the EDCl–NHS-mediated synthetic pathway. Further research on the non-covalent PEI attachment is currently underway.
4. Conclusions
The modification of polyethylenimine and conjugation to carbon-encapsulated iron nanoparticles was presented. Firstly, the PEI derivatives were synthesized by two synthetic strategies: (i) the amide-type reaction using succinic acid anhydride (PEI–SA) and (ii) reductive amination using p-formylbenzoic acid (PEI–pFBA). The reaction yield was found to be between ca. 50 and 57%. The NH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH
NH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio value for the PEI 25 kDa products is 1
N ratio value for the PEI 25 kDa products is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.62
1.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.32 and 1
1.32 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.69
1.69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.39, for PEI25k–SA and PEI25k–pFBA, respectively (the ratio for pristine PEI 25 kDa is 1
1.39, for PEI25k–SA and PEI25k–pFBA, respectively (the ratio for pristine PEI 25 kDa is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12
1.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.89). Those values show that carboxylic functionalities were successfully introduced onto the PEI surface. The structures of the polymer derivatives were analyzed using spectroscopic methods (NMR and FT-IR). Moreover, the measured zeta potential of PEI 25 kDa and its derivatives suggested that the obtained modification level (the amount of introduced functionalities) does not significantly reduce the PEI capability to bind other biomolecules and should provide lower toxicity of the polymer. Next, PEI and its derivatives were conjugated with CEINs containing a shorter or longer surface linker, by a carbodiimide–amine type reaction between primary amino moieties of PEI and carboxylic functionalities onto magnetic nanoparticles. The success of the conjugation was confirmed by FT-IR spectroscopy, thermogravimetry and transmission electron microscopy. The PEI amount in the conjugates was between 6.50% and 52.36%. In most cases, a higher polymer content in the conjugate was found for CEINs with a longer surface linker and PEI of a high molecular weight. Moreover, the obtained conjugates did not contain ‘free’ carboxylic moieties on the CEINs surface. A very good dispersion stability in aqueous media was found for samples containing the longer surface linker. Finally, the non-covalent adsorption phenomenon was investigated. Strong electrostatic interactions between PEI amino moieties and carboxylic moieties onto carbon encapsulates were observed, whilst PEI adsorption was not observed onto pristine (‘linker-free’) CEINs. We claim that the possibility of PEI adsorption, while using the covalent-type conjugation technique, was reduced to the absolute minimum by using a large excess of coupling reagents and an appropriate synthetic procedure. In conclusion, our study sheds a new light onto the conjugation technique of PEI and its derivatives for a promising nanocarrier and drug-contrast candidate – carbon-encapsulated iron nanoparticles. Both PEIs and CEINs offer some interesting and theranostic features, so their potential conjugates can be very useful i.e. in MRI-based molecular diagnostics and targeted anti-cancer therapies.
0.89). Those values show that carboxylic functionalities were successfully introduced onto the PEI surface. The structures of the polymer derivatives were analyzed using spectroscopic methods (NMR and FT-IR). Moreover, the measured zeta potential of PEI 25 kDa and its derivatives suggested that the obtained modification level (the amount of introduced functionalities) does not significantly reduce the PEI capability to bind other biomolecules and should provide lower toxicity of the polymer. Next, PEI and its derivatives were conjugated with CEINs containing a shorter or longer surface linker, by a carbodiimide–amine type reaction between primary amino moieties of PEI and carboxylic functionalities onto magnetic nanoparticles. The success of the conjugation was confirmed by FT-IR spectroscopy, thermogravimetry and transmission electron microscopy. The PEI amount in the conjugates was between 6.50% and 52.36%. In most cases, a higher polymer content in the conjugate was found for CEINs with a longer surface linker and PEI of a high molecular weight. Moreover, the obtained conjugates did not contain ‘free’ carboxylic moieties on the CEINs surface. A very good dispersion stability in aqueous media was found for samples containing the longer surface linker. Finally, the non-covalent adsorption phenomenon was investigated. Strong electrostatic interactions between PEI amino moieties and carboxylic moieties onto carbon encapsulates were observed, whilst PEI adsorption was not observed onto pristine (‘linker-free’) CEINs. We claim that the possibility of PEI adsorption, while using the covalent-type conjugation technique, was reduced to the absolute minimum by using a large excess of coupling reagents and an appropriate synthetic procedure. In conclusion, our study sheds a new light onto the conjugation technique of PEI and its derivatives for a promising nanocarrier and drug-contrast candidate – carbon-encapsulated iron nanoparticles. Both PEIs and CEINs offer some interesting and theranostic features, so their potential conjugates can be very useful i.e. in MRI-based molecular diagnostics and targeted anti-cancer therapies.
Acknowledgements
This work was financially supported partially by Warsaw University of Technology and GEMNS project granted in the European Union’s Seventh Framework Programme under frame of the ERA-NET EuroNanoMed II (European Innovative Research and Technological Development Projects in Nanomedicine).References
- H. van den Berg, J. N. van den Anker and J. H. Beijnen, Cancer Treat. Rev., 2012, 38, 3–26 CrossRef CAS PubMed.
- E. Chatelut, J. P. Delord and P. Canal, Invest. New Drugs, 2003, 21, 141–148 CrossRef CAS.
- N. Hedhli and K. S. Russell, Curr. Hypertens. Rep., 2010, 12, 411–417 CrossRef CAS PubMed.
- Y. Li, T. Lin, Y. Luo, Q. Liu, W. Xiao, W. Guo, D. Lac, H. Zhang, C. Feng, S. Wachsmann-Hogiu, J. H. Walton, S. R. Cherry, D. J. Rowland, D. Kukis, C. Pan and K. S. Lam, Nat. Commun., 2014, 5, 4712 CrossRef CAS PubMed.
- T. Lammers, F. Kiessling, W. E. Hennink and G. Storm, J. Controlled Release, 2012, 161, 175–187 CrossRef CAS PubMed.
- E. K. Lim, T. Kim, S. Paik, S. Haam, Y. M. Huh and K. Lee, Chem. Rev., 2015, 115, 327–394 CrossRef CAS PubMed.
- D. Peer, J. M. Karp, S. Hong, O. C. Farokhazad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- F. Valentini, M. Carbone and G. Palleshi, Anal. Bioanal. Chem., 2013, 405, 451–465 CrossRef CAS PubMed.
- Z. Fan, P. P. Fu, H. Yu and P. C. Ray, J. Food Drug Anal., 2014, 22, 3–17 CrossRef CAS PubMed.
- M. S. Muthu, D. T. Leong, L. Mei and S. S. Feng, Theranostics, 2014, 4, 660–677 CrossRef CAS PubMed.
- T. Moore, H. Chen, R. Morrison, F. Wang, J. N. Anker and F. Alexis, Mol. Pharm., 2014, 11, 24–39 CrossRef CAS PubMed.
- M. Das, S. R. Datir, R. P. Singh and S. Jain, Mol. Pharm., 2013, 10, 2543–2557 CrossRef CAS PubMed.
- S. Mura and P. Couvreur, Adv. Drug Delivery Rev., 2012, 64, 1394–1416 CrossRef CAS PubMed.
- Z. P. Xu, Q. H. Zengb, G. Q. Lua and A. B. Yub, Chem. Eng. Sci., 2006, 61, 1027–1040 CrossRef CAS PubMed.
- M. Orecchioni, R. Cabizza, A. Bianco and L. G. Delogu, Theranostics, 2015, 5, 710–723 CrossRef CAS PubMed.
- S. Afreen, K. Muthoosamy, S. Manickam and U. Hashim, Biosens. Bioelectron., 2015, 63, 354–364 CrossRef CAS PubMed.
- S. Bosi, T. Da Ros, G. Spalluto and M. Prato, Eur. J. Med. Chem., 2003, 38, 913–923 CrossRef CAS PubMed.
- J. Borysiuk, A. Grabias, J. Szczytko, M. Bystrzejewski, A. Twardowski and H. Lange, Carbon, 2008, 46, 1693–1701 CrossRef CAS PubMed.
- M. Bystrzejewski, A. Huczko and H. Lange, Sens. Actuators, B, 2005, 109, 81–85 CrossRef CAS PubMed.
- I. P. Grudzinski, M. Bystrzejewski, M. A. Cywinska, A. Kosmider, M. Poplawska, A. Cieszanowski, Z. Fijalek and A. Ostrowska, Colloids Surf., B, 2014, 117, 135–143 CrossRef CAS PubMed.
- I. P. Grudzinski, M. Bystrzejewski, M. A. Cywilska, A. Kosmider, M. Poplawska, A. Cieszanowski and A. Ostrowska, J. Nanopart. Res., 2013, 15, 1835 CrossRef PubMed.
- I. P. Grudzinski, M. Bystrzejewski, M. A. Cywinska, A. Kosmider, M. Poplawska, A. Cieszanowski, Z. Fijalek, A. Ostrowska and A. Parzonko, J. Appl. Toxicol., 2014, 34, 380–394 CrossRef CAS PubMed.
- B. Behnam, W. T. Shier, A. H. Nia, K. Abnous and M. Ramezani, Int. J. Pharm., 2013, 454, 204–215 CrossRef CAS PubMed.
- S. Polizu, O. Savadogo, P. Poulin and L. Yahia, J. Nanosci. Nanotechnol., 2006, 6, 1883–1904 CrossRef CAS PubMed.
- Z. Liu, X. Sun, N. Nakayama-Ratchford and H. Dai, ACS Nano, 2007, 1, 55–56 Search PubMed.
- H. Ali-Boucetta, K. T. Al-Jamal, D. McCarthy, M. Prato, A. Bianco and K. Kostarelos, Chem. Commun., 2008, 459–461 RSC.
- C. Klumpp, K. Kostarelos, M. Prato and A. Bianco, Biochim. Biophys. Acta, 2006, 1758, 404–412 CrossRef CAS PubMed.
- S. W. Kim, T. Kim, Y. S. Kim, H. S. Choi, H. J. Lim, S. J. Yang and C. R. Park, Carbon, 2012, 50, 3–33 CrossRef CAS PubMed.
- S. Eigler and A. Hirsch, Angew. Chem., Int. Ed., 2014, 53, 2–21 CrossRef PubMed.
- M. Quintana, E. Vazquez and M. Prato, Acc. Chem. Res., 2013, 46, 138–148 CrossRef CAS PubMed.
- M. Poplawska, M. Bystrzejewski, I. P. Grudzinski, M. A. Cywinska, J. Ostapko and A. Cieszanowski, Carbon, 2014, 74, 180–194 CrossRef CAS PubMed.
- M. Popławska, G. Z. Zukowska, S. Cudziło and M. Bystrzejewski, Carbon, 2010, 48, 1312–1320 CrossRef PubMed.
- S. K. Samal, M. Dash, S. van Vlierberghe, D. L. Kaplan, E. Chiellini, C. van Blitterswijk, L. Moronid and P. Dubruel, Chem. Soc. Rev., 2012, 41, 7147–7194 RSC.
- R. Soleyman and M. Adeli, Polym. Chem., 2015, 6, 10–24 RSC.
- O. M. Merkel, D. Librizzi, A. Pfestroff, T. Schurrat, K. Buyens, N. N. Sanders, S. C. de Smedt, M. Béhé and T. Kissel, J. Controlled Release, 2009, 138, 148–159 CrossRef CAS PubMed.
- H. Ragelle, G. Vandermeulen and V. Préat, J. Controlled Release, 2013, 172, 207–218 CrossRef CAS PubMed.
- A. F. M. El-Mahdy, T. Shibata, T. Kabashima, Q. Zhu and M. Kai, RSC Adv., 2015, 5, 32775–32785 RSC.
- S. A. Meenach, Y. J. Kim, K. J. Kauffman, N. Kanthamneni, E. M. Bachelder and K. M. Ainslie, Mol. Pharm., 2012, 9, 290–298 CrossRef CAS PubMed.
- J. Zhao, C. Lu, X. He, X. Zhang, W. Zhang and X. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 2607–2615 CAS.
- H. Wu, H. Shi, H. Zhang, X. Wang, Y. Yang, C. Yu, C. Hao, J. Du, H. Hu and S. Yang, Biomaterials, 2014, 35, 5369–5380 CrossRef CAS PubMed.
- M. Shen, S. H. Wang, X. Shi, X. Chen, Q. Huang, E. J. Petersen, R. A. Pinto, J. R. Baker Jr and W. J. Weber Jr, J. Phys. Chem. C, 2009, 113, 3150–3156 CAS.
- S. Foillard, G. Zuber and E. Doris, Nanoscale, 2011, 3, 1461–1464 RSC.
- M. Zhang, Y. Li, Z. Su and G. Wei, Polym. Chem., 2015, 6, 6107–6124 RSC.
- L. Feng, X. Yang, X. Shi, X. Fan, R. Peng, J. Wang and Z. Liu, Small, 2013, 9, 1989–1997 CrossRef CAS PubMed.
- G. Wei, R. Dong, D. Wang, L. Feng, S. Dong, A. Song and J. Hao, New J. Chem., 2014, 38, 140–145 RSC.
- L. Feng, S. Zhang and Z. Liu, Nanoscale, 2011, 3, 1252–1257 RSC.
- A. Zintchenko, A. Philipp, A. Dehshahri and E. Wagner, Bioconjugate Chem., 2008, 19, 1448–1455 CrossRef CAS PubMed.
- S. Nimesh, A. Aggarwal, P. Kumar, Y. Singh, K. C. Gupta and R. Chandra, Int. J. Pharm., 2007, 337, 265–274 CrossRef CAS PubMed.
- P. Y. Teo, C. Yang, J. L. Hedrick, A. C. Engler, D. J. Coady, S. Ghaem-Maghami, A. J. T. George and Y. Y. Yang, Biomaterials, 2013, 34, 7971–7979 CrossRef CAS PubMed.
- X. Chen, Z. Yuan, X. Yi, R. Zhuo and F. Li, Nanotechnology, 2012, 23, 415602 CrossRef PubMed.
- X. Zhang, Y. Duan, D. Wang and F. Bian, Carbohydr. Polym., 2015, 122, 53–59 CrossRef CAS PubMed.
- W. Tseng and C. Jong, Biomacromolecules, 2003, 4, 1277–1284 CrossRef CAS PubMed.
- J. Wang, B. Dou and Y. Bao, Mater. Sci. Eng., C, 2014, 34, 98–109 CrossRef PubMed.
- S. Wen, F. Zheng, M. Shen and X. Shi, J. Appl. Polym. Sci., 2013, 128, 3807–3813 CrossRef CAS PubMed.
- H. Petersen, P. M. Fechner, D. Fischer and T. Kissel, Macromolecules, 2002, 35, 6867–6874 CrossRef CAS.
- K. S. Siu, D. Chen, X. Zheng, X. Zhang, N. Johnston, Y. Liu, K. Yuan, J. Koropatnick, E. R. Gilles and W.-P. Min, Biomaterials, 2014, 35, 3435–3442 CrossRef CAS PubMed.
- X. Cao, Z. Li, X. Song, X. Cui, P. Cao, H. Liu, F. Cheng and Y. Chen, Eur. Polym. J., 2008, 44, 1060–1070 CrossRef CAS PubMed.
- H. Liu, Z. Shen, S.-E. Stiriba, Y. Chen, W. Zhang and L. Wei, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 4165–4173 CrossRef CAS PubMed.
- H. Lv, S. Zhang, B. Wang, S. Cui and J. Yan, J. Controlled Release, 2006, 114, 100–109 CrossRef CAS PubMed.
- Y. Gao and I. Kyratzis, Bioconjugate Chem., 2008, 19, 1945–1950 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17912b |
| This journal is © The Royal Society of Chemistry 2015 |