Fabrication of translucent nanoceramics via a simple filtration method
Abstract
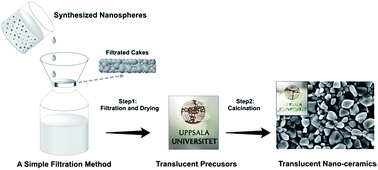
Generally, particle packing density, grain size and morphology are the important factors that affect the transparency of ceramics. In order to achieve better transparency of ceramics, efforts should be developed to eliminate or minimize light scattering or absorption. Therefore the porosity and size of crystals in a ceramic body should be strictly controlled. Typical transparent ceramics are fabricated by pressure-assisted sintering techniques such as hot isostatic pressing (HIP), spark plasma sintering (SPS), and pressure-less sintering (PLS). However, a simple energy efficient production method remains a challenge. In this study, we describe a simple fabrication process via a facile filtration system that can fabricate translucent hydroxyapatite based ceramics. The translucent pieces yielded from filtration exhibit optical transmittance that was confirmed by UV spectroscopy. Briefly, the morphology and size of ceramic nanoparticles, filtration pressure and filtration time are important parameters to be discussed. Compared with different hydroxyapatite nanoparticles, spherical nanoparticles easily form a densely packed structure, followed by sintered ceramics. When the strontium content in HA increases, the morphology of HA changes from nano-spheres to nano-rods, following a decrease in transparency. A pressure filtration model combining Darcy's law and the Kozeny–Carman relation has been discussed to simulate and explain why the translucent ceramics can be fabricated via such a simple process. This method could be further applied to prepare other translucent functional ceramics by controlling the size and morphology of ceramic particles.

 Please wait while we load your content...
Please wait while we load your content...