Detection of nonfluorescent cyhalothrin in honey by a spheral SiO2-based particle coating with thin fluorescent molecularly imprinted polymers film†
Abstract
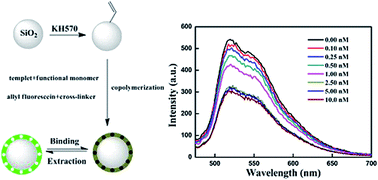
In this study, we report a general protocol for making core–shell SiO2@KH570-MIP based on the surface modification of SiO2 beads for the selective detection of ultra trace cyhalothrin. We first prepared the fluorescent surface molecularly imprinted polymer (SMIP) spheres via copolymerization of acrylamide with allyl fluorescein in the presence of cyhalothrin to form recognition sites. The experimental results showed that the fluorescence quenching of SiO2@KH570-MIP for cyhalothrin was much higher than that of the structural analogue composite, which illustrated good recognition capacity and selectivity of SiO2@KH570-MIP for the template. In addition, a linear relationship could be obtained at a lower concentration range of 0–2.5 nM with a correlation coefficient of 0.99698 described by the Stern–Volmer equation. The results of practical detection suggest that the developed method satisfactorily determines cyhalothrin in honey samples. This study therefore demonstrated the potential of SiO2-based SMIP spheres for the recognition and detection of cyhalothrin in food.


 Please wait while we load your content...
Please wait while we load your content...