Fabrication and application of non-rare earth red phosphors for warm white-light-emitting diodes†
Abstract
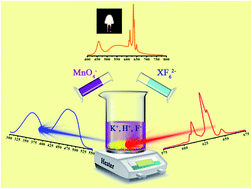
In this work, a facile and efficient method for the preparation of K2XF6:Mn4+ (X = Si, Ti and Ge) red phosphors is reported. They were synthesized by adding KF to precipitate a warm mixed solution with XO2, KMnO4 and HF as raw materials. After the doping of Mn4+, although the obtained products exhibit irregular micro-sized particulate morphologies, they present the same single phases as their matrices and no impurities can be found. The optical properties of the fluoride complexes were investigated using photo-luminescence spectroscopy, diffuse reflectance spectroscopy, and luminescence decay curves. The complexes presented bright red emission under blue light illumination, and warm white-light-emitting diodes with low correlated color temperature, high color rendering index and high luminous efficiency, with values of 3156 K, 84.9 and 138.4 lm W−1, respectively, were achieved by coating a mixture of the red phosphor with commercial Y3Al5O12:Ce3+ on blue-GaN chips.

 Please wait while we load your content...
Please wait while we load your content...