Highly selective fluorescence detection of Pd2+/4+ species based on a catalyzed aromatic Claisen rearrangement†
Xuejing Lia,
Huanhuan Huanga,
Yuqing Zhua,
Hong Zhao*a and
Zhifei Wang*ab
aSchool of Chemistry and Chemical Engineering, Southeast University, Nanjing, 211189, China. E-mail: zfwang@seu.edu.cn; zhaohong@seu.edu.cn
bJiangsu Key Laboratory of Advanced Metallic Materials, Nanjing, 211189, China
First published on 30th November 2015
Abstract
A highly selective chemodosimeter based on 1,8-naphthalimide for Pd2+/4+ species via a Claisen rearrangement was developed, which not only discriminated Pd from competing Pt species, but also distinguished Pd in an oxidized state without altering its oxidation states from Pd0. Under optimized reaction conditions, the detection limit can reach 1.4 μM for Pd2+.
As one of the rare transition metals, palladium plays an important role in many fields, such as electronics, dentistry, medicine, jewellery, and as a catalyst in synthetic chemistry or automotive catalytic converters.1 Such increase in its industrial use has raised great concern about its emission into the biosphere and the further toxicological effects on human health.2 Consequently, the detection of palladium is of great interest and importance to many chemists, biologists and environmentalists. In the past few years, many analytical methods, including inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectrometry (AAS), plasma emission spectroscopy (ICP-AES), X-ray fluorescence (XRF), etc., have been developed for the detection of palladium.3 However, these methods often require a complicated sample pretreatment, sophisticated instrumentation, and highly skilled individuals. Meanwhile, the development of fluorescence methods based on chemodosimeters for qualitatively and quantitatively detecting palladium species has blossomed in recent years due to their low cost, convenient operation, and high accuracy.4
Up to now, the fluorescent sensing systems for palladium species reported were mostly based on the following three mechanisms: a Pd-coordination reaction,5 a Pd-triggered cleavage reaction1,6–13 and a Pd-catalyzed Claisen rearrangement reaction.14 In the first case, the fluorescent probes provide well-organized coordination geometry for palladium ions. Because the coordination capability of ligands for palladium ions is significantly influenced by the solvent conditions such as a change in pH value, their selectivity may be a major issue for such coordination based sensing systems. In the second case, Koide et al. developed a chemodosimeter based on the Pd-triggered Tsuji–Trost reaction. In this system, an allylated fluorescein, which is nonfluorescent, undergoes catalytic deallylation or depropargylation with Pd0 generated in situ from a Pd2+ species to regenerate the fluorescein. However, most of these types of chemodosimeters require an initial conversion of Pd2+/4+ to Pd0 using a reducing agent such as PPh3, TFP (tri-2-furylphosphine) or TFP–NaBH4. To overcome the above limitation, in 2008, Koide et al.14 further presented the third type; a Pittsburgh Green based chemodosimeter using a Claisen rearrangement reaction, in which the Pd species could be detected in an oxidation state-specific manner without altering oxidation states. Although there were some improvements on the detection of Pd2+/4+, this method couldn’t discriminate Pd2+/4+ from Pt4+ species. As one of the platinum-group elements, palladium shows a similar catalytic effect to platinum and is usually used in automotive catalytic converters together with platinum,14 which indicates that any interference from platinum is a disadvantage from the viewpoint of selective detection. Therefore, the search for selective chemodosimeters for Pd species in a oxidation state is still a challenging task.
On the other hand, 1,8-naphthalimide with an electron donor and an acceptor group is characteristic of an internal charge transfer (ICT) chromophore, and its fluorescence property is highly sensitive to the polarity of the local environment. So far, naphthalimide derivatives have been widely used as fluorescent chemodosimeters.15 Inspired by the successful application of the Claisen rearrangement reaction in the Pittsburgh Green based chemodosimeter, we hope to further investigate the feasibility of this strategy on the design of one based on naphthalimide.
Based on the above considerations, probe 1 was synthesized in four steps from commercially available 4-bromo-1,8-naphthalic anhydride in a satisfactory yield (Scheme 1, ESI Fig. S1–S4†). After being treated with Pd2+/4+ species under mild condition, probe 1 is expected to be converted to compound 5 by a Claisen rearrangement reaction.
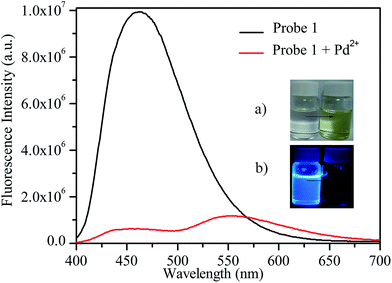
We firstly studied the spectroscopic properties of probe 1 in Na2CO3/NaHCO3 buffer (pH = 10) solution. As shown in Fig. 1, in the absence of Pd2+, probe 1 (12.5 μM) showed a relatively short emission wavelength with a maximum at 461 nm (blue), as a result of the electron-withdrawing effect of the amide group. After being treated with Na2PdCl4 (25 μM) at 50 °C for 4 h, the fluorescence intensity of probe 1 at 461 nm decreased dramatically, and a new weak emission band appeared at 552 nm. From the inset in Fig. 1, it can be seen that the colour change in the solutions, ranging from colourless to yellow, even could be distinguished by the naked eye which is also verified by the change on the corresponding adsorption spectra (Fig. S6†). To investigate the sensing mechanism of probe 1 for palladium species, after separation and purification, the reaction product of probe 1 with Pd2+, which was the product of typical Claisen rearrangement reaction, was confirmed by 1H NMR and 13C NMR (Fig. S5†). In addition, the characteristic fluorescence spectrum of a standard pure compound 5 also supports this analysis (Fig. S9†). Similarly to the monoallyl ether Pittsburgh Green,14 a Claisen rearrangement of probe 1 with Pd2+ may proceed through the mechanism shown in Scheme S1.†
As we know, a Claisen rearrangement reaction is generally influenced by various factors,16 such as temperature, pH and reaction time. To achieve an optimal analytical performance, the experimental conditions for the fluorescence emission were further optimized through testing the temperature from 30 to 70 °C, pH from 4 to 14, and the reaction time from 0 to 5 h. As shown in Fig. S10,† the fluorescence intensity at 461 nm decreased considerably when the temperature was higher than 50 °C, which indicates that 50 °C was the critical temperature for the Claisen rearrangement reaction. For the effect of pH on the fluorescence emission (Fig. S11†), it can be clearly seen that the reaction occurred at pH 7–14 and the corresponding fluorescence intensity obviously decreased in this range. Fig. S12† shows the fluorescence intensity at 461 nm in the presence of Pd2+ at 50 °C as a function of incubation time, and it can be found that the fluorescence intensity decreased quickly within 2 h and reached a minimum after 4 h of reaction. According to the above results, in the following reactions, the detection of Pd2+/4+ was performed in Na2CO3/NaHCO3 buffer (pH = 10) at 50 °C with a 4 h incubation time.
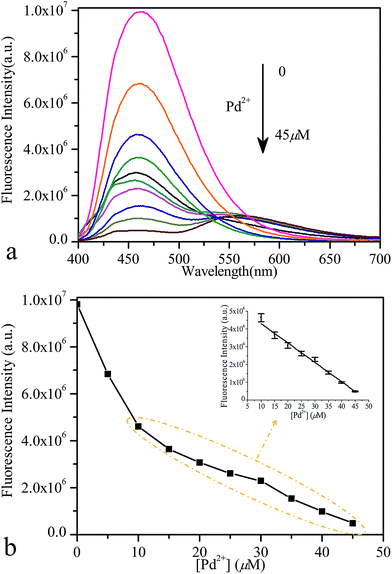
Variations of the fluorescence spectra of probe 1 as a function of Pd2+ concentration were measured to evaluate its sensing behaviour, as shown in Fig. 2a. As expected, upon the increase of Pd2+ concentration (0–45 μM), a gradual decrease in fluorescence intensity at 461 nm was observed (Fig. 2b). In Fig. 2b inset, it can be seen that the sensor exhibited a linear response toward Pd2+ in the concentration range from 10 to 45 μM. The linear equation was y = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 387
387![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 − 109
520 − 109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106x, and the linear relative coefficient was R2 = 0.995. The detection limit under these conditions was determined as 1.4 μM with a signal-to-background ratio (S/B) of 3, which was slightly higher than the result reported by Koide et al. using a similar detection mechanism.14
106x, and the linear relative coefficient was R2 = 0.995. The detection limit under these conditions was determined as 1.4 μM with a signal-to-background ratio (S/B) of 3, which was slightly higher than the result reported by Koide et al. using a similar detection mechanism.14
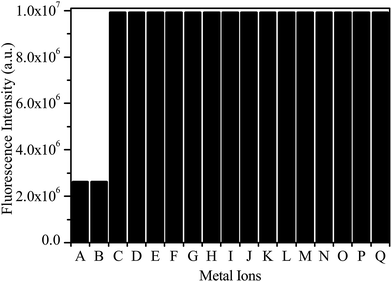
The selectivity of probe 1 was further examined by the fluorescence responses of the probe toward different metal ions. As shown in Fig. 3, the fluorescence at 461 nm of the probe 1 decreased only in the cases of Pd2+ and Pd4+; other metal ions such as Pd0, Pt0, Pt2+, Pt4+, Na+, Ca2+, Mn2+, Co2+, Cu2+, Zn2+, Cd2+, Ni2+, Au3+, Ag+ and Fe3+ had no effect on the emission of probe 1. This result clearly demonstrated that probe 1 had a high selectivity and could discriminate Pd2+/4+ species from both Pd0 and Pt0/2+/4+ species, which was better than the result reported by Koide et al.,14 in which the Pittsburgh Green based chemodosimeter couldn’t discriminate Pd2+/4+ from Pt4+ via a similar Claisen rearrangement reaction. Considering the fact that the catalysis of Claisen rearrangement is complex and greatly depends on the substance and reaction parameters,16 the above result is understandable. In our system, 1,8-naphthalimide was used as the chromophore instead of a xanthene moiety. To the best of our knowledge, it is the first report in which the resulting chemodosimeter for Pd2+/4+ species has such a high selectivity.
To demonstrate the practical application of probe 1, we conducted experiments to detect residual Pd ions in a typical Heck reaction. The cross coupling of acrylic acid with iodobenzene catalyzed by Pd0 was performed according to the reported method.17 Many studies had indicated that the activity of Pd0 that decreased in re-use was probably due to the leaching of palladium into the solution in the state of Pd0 or Pd2+. To verify the above speculation, probe 1 was added to the solution after the separation of Pd0 and product. After being treated at 50 °C for 4 h, the fluorescence intensity of the resulting solution decreased significantly. According to the decrement in fluorescence intensity, the concentration of Pd2+ was about 33.5 μM. Therefore, it can be concluded that the leaching of Pd into the solution in an oxidized state was one of the causes that leads to the decrease of the activity of the catalyst Pd0 in the Heck reaction.
Due to our successful detection for Pd2+/4+ with no Pt-interference, we next performed a test for a three-way catalyst (TWC) sample. A TWC was used in this case because it contains both palladium and platinum simultaneously.18 After initial treatment of the TWC with 5% HNO3, the concentrations of Pdn+ and Ptn+ were about 15 μM and 25 μM, respectively. As shown in Fig. S13,† the fluorescence intensity at 461 nm of the TWC corresponded to 15.5 μM of Pd2+, which further supported the above result that any interference from Ptn+ had no effect on the detection of Pd2+/4+ using probe 1.
Conclusions
In conclusion, we have synthesized a highly selective chemodosimeter based on 1,8-naphthalimide for Pd2+/4+ species. The recognition mechanism is via a Claisen rearrangement reaction. The resulting fluorescent probe not only discriminated Pd from competing Pt species, but also distinguished Pd in an oxidized state without altering its oxidation states from Pd0. With the experimental conditions optimized, the probe exhibits a linear response for Pd2+ from 10 to 45 μM, with a detection limit of 1.4 μM.Acknowledgements
This research is financially supported by the State Key Basic Research Program of the PRC (2014CB744501), Jiangsu province natural science foundation (BK20141332), Jiangsu provincial financial support of fundamental conditions and science and technology for people’s livelihood for Jiangsu key laboratory of advanced metallic materials (BM2007204) and the Fundamental Research Funds for the Central Universities.Notes and references
- M. Santra, S. K. Ko, I. Shin and K. H. Ahn, Chem. Commun., 2010, 46, 3964–3966 RSC.
- (a) T. Z. Liu, S. D. Lee and R. S. Bhatnagar, Toxicol. Lett., 1979, 4, 469–473 CrossRef CAS; (b) J. C. Wataha and C. T. Hanks, J. Oral Rehabil., 1996, 23, 309–320 CrossRef CAS.
- (a) B. Dimitrova, K. Benkhedda, E. Ivanova and F. Adams, J. Anal. At. Spectrom., 2004, 19, 1394–1396 RSC; (b) C. Locatelli, D. Melucci and G. Torsi, Anal. Bioanal. Chem., 2005, 382, 1567–1573 CrossRef CAS; (c) M. K. Van, A. Smekens, M. Behets, P. Kazandjian and G. R. Van, Anal. Chem., 2007, 79, 6383–6389 CrossRef PubMed.
- (a) H. Li, J. Fan and X. Peng, Chem. Soc. Rev., 2013, 42, 7943–7962 RSC; (b) J. Du, M. Hu, J. Fan and X. Peng, Chem. Soc. Rev., 2012, 41, 4511–4535 RSC; (c) K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564–4601 CrossRef CAS PubMed; (d) Y. Ding, Y. Tang, W. Zhu and Y. Xie, Chem. Soc. Rev., 2015, 44, 1101–1112 RSC; (e) Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 14–29 Search PubMed; (f) L. Zhu, A. H. Younes, Z. Yuan and R. J. Clark, J. Photochem. Photobiol., A, 2015, 31, 11–15 Search PubMed; (g) L. Zhu, Z. Yuan, J. T. Simmons and K. Sreenath, RSC Adv., 2014, 4, 20398–20440 RSC.
- (a) S. G. Sun, B. Qiao, N. Jiang, J. T. Wang, S. Zhang and X. J. Peng, Org. Lett., 2014, 16, 1132–1135 CrossRef CAS PubMed; (b) H. L. Li, J. L. Fan, J. J. Du, K. X. Guo, S. G. Sun and X. J. Liu, et al., Chem. Commun., 2010, 46, 1079–1081 RSC; (c) H. L. Li, J. L. Fan, X. J. Liu, S. G. Sun and X. J. Peng, et al., Chem. Res. Chin. Univ., 2010, 31, 1725–1728 CAS; (d) H. Kim, K. S. Moon, S. Shim and J. Tae, Chem.–Asian J., 2011, 6, 1987–1991 CrossRef CAS PubMed; (e) L. P. Duan, Y. F. Xu and X. H. Qian, Chem. Commun., 2008, 47, 6339–6341 RSC.
- F. L. Song, A. L. Garner and K. Koide, J. Am. Chem. Soc., 2007, 129, 12354–12355 CrossRef CAS PubMed.
- A. L. Garner and K. Koide, Chem. Commun., 2009, 1, 86–88 RSC.
- A. L. Garner and K. Koide, Chem. Commun., 2009, 1, 83–85 RSC.
- J. Jiang, H. Jiang, W. Liu, X. L. Tang and X. Zhou, et al., Org. Lett., 2011, 13, 4922–4925 CrossRef CAS.
- B. Zhu, C. Gao, Y. Zhao, C. Liu, Y. Li and Q. Wei, et al., Chem. Commun., 2011, 47, 8656–8658 RSC.
- W. Liu, J. Jiang, C. Chen, X. Tang, J. Shi and P. Zhang, et al., Inorg. Chem., 2014, 53, 12590–12594 CrossRef CAS.
- W. R. Kitley, M. Santa, R. A. Cloyd and L. M. Wysocki, Chem. Commun., 2015, 51, 8520–8523 RSC.
- K. Wang, G. Lai, Z. Li, M. Liu and Y. Shen, Tetrahedron, 2015, 71, 7874–7878 CrossRef CAS.
- A. L. Garner and K. Koide, J. Am. Chem. Soc., 2008, 130, 16472–16473 CrossRef CAS PubMed.
- (a) R. M. Duke, E. B. Veale, F. M. Pfeffer, P. E. Kruger and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3936–3953 RSC; (b) Z. C. Xu, K. H. Baek, H. N. Kim, J. N. Cui and X. H. Qian, et al., J. Am. Chem. Soc., 2010, 132, 601–610 CrossRef CAS; (c) L. Cui, Y. Zhong, W. P. Zhu, Y. F. Xu and X. H. Qian, Chem. Commun., 2010, 46, 7121–7123 RSC; (d) Z. C. Xu, J. Y. Yoon and D. R. Spring, Chem. Commun., 2010, 46, 2563–2565 RSC.
- K. C. Majumdar, S. Alam and B. Chattopadhyway, Tetrahetron, 2008, 64, 597–643 CrossRef CAS.
- Z. F. Wang, P. F. Xiao, B. Shen and N. Y. He, Colloids Surf., A, 2006, 276, 116–121 CrossRef CAS.
- R. M. Heck and R. J. Farrauto, Appl. Catal., A, 2001, 221, 443–457 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17831b |
| This journal is © The Royal Society of Chemistry 2015 |