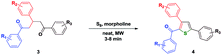A direct green route towards the synthesis of 2-aroyl-3,5-diarylthiophenes from 1,5-diketones†
Rengasamy Chithiravela,
Kandasamy Rajagurua,
Shanmugam Muthusubramanian*a and
Nattamai Bhuvaneshb
aDepartment of Organic Chemistry, School of Chemistry, Madurai Kamaraj University, Madurai-625 021, India. E-mail: muthumanian2001@yahoo.com
bX-ray Diffraction Laboratory, Department of Chemistry, Texas A & M University, College Station, Texas 77842, USA
First published on 7th October 2015
Abstract
The synthesis of 2-aroyl-3,5-diarylthiophenes has been achieved from various 1,5-diketones with elemental sulphur and morpholine under efficient microwave conditions. The reaction follows a green protocol involving solvent-free conditions, short reaction time, and simple work-up avoiding chromatographic purification.
Introduction
The synthesis of heterocyclic motifs with one or more heteroatoms from acyclic counterparts has traditionally attracted attention.1 Thiophene is an important class of heterocycle forming the core of several compounds from natural and synthetic sources with widespread biological properties with promising pharmacological characteristics.2,3 Most of the substituted thiophenes find numerous applications in modern drug design,4 biodiagnostics,5 electronic and optoelectronic devices,6 conductivity-based sensors,7 self-assembled superstructures,8 and dye chemistry.9 Several synthetic methods have been developed to generate trisubstituted thiophenes from functionalized carbonyl systems.10 The classical strategy for the synthesis of thiophenes involves the reaction of phosphorous sulphides such as Lawesson's reagent or bis-(trimethylsilyl)sulphide with 1,4-dicarbonyl systems.11 Still the methods for constructing of di- or tri-substituted thiophene derivatives are meagre among the existing synthetic strategies.12 Several transition metal catalyzed cross-couplings as well as C–S bond formation reactions have been employed for the synthesis of trisubstituted thiophenes.13 Multicomponent and domino reactions have gained tremendous attention for the construction of numerous complex molecules.14 In continuation of our efforts in the generation of heterocycles from 1,5-diketones and other functionalised acyclic systems,15 we planned to synthesize bisthiophenes under Gewald conditions. Interestingly the reaction has resulted in a different product. The Gewald reaction is the most convenient and well established protocol for the synthesis of 2-aminothiophenes16 and it involves the three-component domino reaction of a ketone with an activated nitrile and elemental sulfur in the presence of a base, this transformation being a remarkable one as the elemental sulphur is incorporated in the ring. 2-Aminothiophenes serve as important intermediates in dye preparation and drug formulations and as the basic framework for a variety of natural products.17Results and discussion
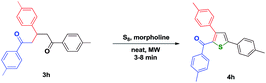
The plan of this investigation was to generate bis-thiophene from the Gewald reaction of 1,3,5-triaryl-1,5-diketones and the interesting outcome is described here. The 1,5-diketones are very ideal to Gewald reaction with two different ends being ready to get cyclised. The 1,5-diketones 3 for the present investigation have been prepared by slightly modifying the reported procedure in a greener route.18 Out of the diketones synthesised, 3a–3e and 3m–3o have not been reported so far. It must be indicated that a preliminary attempt to get the Gewald product from 1,3,5-triphenyl-1,5-diketone was a failure from our group.15a Now by changing the conditions, the reaction has been revisited. In a typical procedure to generate the bisthiophene, a mixture of 1,5-diketone 3h (R1, R2, R3 = 4-CH3) (1.0 equiv.), malanonitrile (2.0 equiv.) and stoichiometric amount of sulfur (2.2 equiv.) in the presence of diethylamine (4.0 equiv.) was subjected to microwave irradiation for 20 minutes at 70 °C under solvent free condition. On completion of the reaction, a white solid was obtained upon washing with water and subsequent recrystallization from ethanol.19 This pure product was expected to be the bisthiophene 4′ by a double-Gewald pathway. But a closer analysis of the spectral data suggested that the desired bisthiophene has not been formed (Scheme 1) and a different product 4h was obtained.Obviously the sulfur has been incorporated in the 1,5-diketone motif and the ring formation has occurred in an intramolecular fashion without involving malanonitrile. Interestingly, in the absence of malanonitrile, the reaction has led to the formation of 2-aroyl-3,5-diarylthiophene 4h exclusively. After this serendipity result, it has been tried to get the expected bisthiophene by optimising the reaction conditions. The screening was carried out with different bases such as triethylamine, piperidine, morpholine, pyridine and DABCO and the stoichiometric amount of sulfur (2.2–2.8 equiv.) by varying the temperature under microwave irradiation for different time durations with no solvent. Increasing the reaction time and the base concentration (upto 10 equiv.) has led to no significant change in the formation 4h and no bisthiophene 4′ has been formed. The clean and neat conversion to 4h has been observed with 2.0 equivalent of morpholine and 1.1 equivalent of elemental sulfur at 110 °C within 5 min at microwave irradiation. Lowering the temperature led to a moderate yield of 65%. There is no significant improvement in the product yield at higher temperature. The 1H NMR spectrum of 4h shows a distinct singlet at 7.37 ppm other than the aryl ring protons of the original substrate. The absence of –NH2 peak is very much conspicuous. The 13C NMR spectrum clearly indicates the presence of a carbonyl group with the signal at 189.4 ppm. The characteristic nitrile carbon peak was not found. In ESI-MS m/z analysis, the mass of the compound 4h has been found to be 383.11. These observations were in agreement with the formation of a 2-aroyl-3,5-diarylthiophene.
Under the optimized conditions (Entry 9, Table 1), a library of thiophenes have been prepared (Table 2) and thus a facile and elegant method to synthesize 3,5-diaryl-2-aroylthiophenes has been revealed in this investigation.
| Entry | Base (equiv.) | Malanonitrile (equiv.) | S8 (equiv.) | Temp. (°C) | Time (min) | Yield % 4h |
|---|---|---|---|---|---|---|
| a Reaction carried out under solvent free condition.b Starting material recovered. | ||||||
| 1 | Diethylamine (4.0) | 2.0 | 2.2 | 70 | 20 | 24 |
| 2 | Triethylamine (4.0) | 2.0 | 2.8 | 70 | 20 | Nilb |
| 3 | Pyridine (10.0) | 2.8 | 2.8 | 110 | 5 | Nilb |
| 4 | Piperidine (4.0) | 2.0 | 2.2 | 110 | 30 | 31 |
| 5 | Piperidine (8.0) | 2.4 | 2.8 | 130 | 30 | 33 |
| 6 | Pyrrolidine (4.0) | 2.2 | 2.2 | 100 | 25 | 38 |
| 7 | Morpholine (4.0) | 2.0 | 2.2 | 80 | 20 | 67 |
| 8 | Morpholine (4.0) | — | 1.1 | 80 | 15 | 65 |
| 9 | Morpholine (2.0) | — | 1.1 | 110 | 5 | 92 |
| 10 | Morpholine (2.0) | — | 1.1 | 150 | 5 | 91 |
| 11 | DABCO (2.0) | — | 1.1 | 110 | 20 | Nilb |
| a Reaction condition: In a 10 mL quartz vial 1,3,5-triarylpentane-1,5-dione 3 (1.0 equiv.), morpholine (2.0 equiv.) and elemental sulfur (1.1 equiv.) was sealed and subjected to microwave irradiation at 110 °C for 5 min. |
|---|
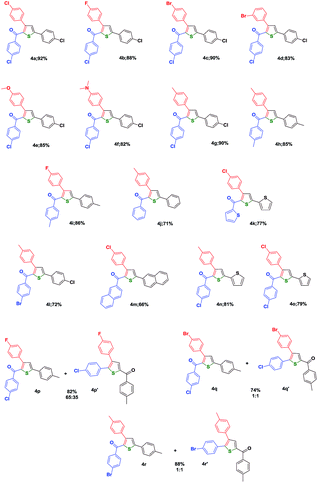 |
When the optimized condition was applied to symmetrical diketones (3a–3k) with identical substituents (R1 = R3), the mode of cyclization could be possible at either of the carbonyl carbons resulting in a single product 4a–4k. If the starting diketone is unsymmetrical (3l, 3n and 3o) with different substituents at 1 and 5 positions (R1≠R3), there is a possibility for the formation of two regioisomeric products. However, the major isomer has been isolated in the pure form in the cases of 4l, 4n and 4o, where the major: minor ratio ranged from 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 to 80
40 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. However, an inseparable mixture of regioisomers has been obtained with 4p (65
20. However, an inseparable mixture of regioisomers has been obtained with 4p (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35), 4q (1
35), 4q (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 4r (1
1) and 4r (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as revealed by the NMR spectra of the mixtures. It is difficult to ascertain which of the regioisomers has been obtained as the major one by 1H NMR and 13C NMR spectral data. However, the regiochemical arrangement for the major isomers of 4l, 4n and 4o shown in Table 2 can be justified vide infra.
1) as revealed by the NMR spectra of the mixtures. It is difficult to ascertain which of the regioisomers has been obtained as the major one by 1H NMR and 13C NMR spectral data. However, the regiochemical arrangement for the major isomers of 4l, 4n and 4o shown in Table 2 can be justified vide infra.
A single crystal X-ray crystallographic study of 4a20 unambiguously (Fig. 1) discloses the structure of the product 4.
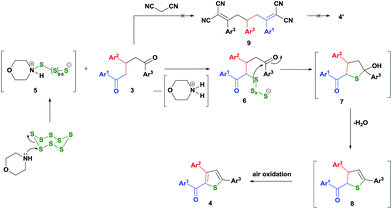
The mechanism for the formation of 3,5-diaryl-2-aroylthiophene 4 has been proposed (Scheme 2) and initially, morpholine polysulfide21 5 has been formed in situ by the activation of elemental sulfur by morpholine. Then this polysulfide removes a proton from the most acidic α-methylene position creating a reactive nucleophilic poly sulphide adduct 6. The anion 6 behaves as a nucleophile attacking the carbonyl carbon forming 7 and the subsequent dehydration results in 8. Upon air oxidation, stable 3,5-diaryl-2-aroylthiophene 4 has been obtained. The formation of Knoevenagel product 1,5-bisarylidene malanonitrile 9 and the traditional Gewald reaction have not occurred even with the higher base concentration.
Regarding the unsymmetrical systems, of the two different ends, that end where a carbanion formation is stabilized will be attacked by the sulphur adduct. If the aroyl group has an electron releasing substituent, then that group will compete for the stabilization of anion and hence the formation of carbanion at that end may be difficult to achieve. This point is well documented in Scheme 3 justifying the proposed structures for the major regioisomers 4l, 4n and 4o. Unfortunately, it was not possible to grow single crystals in these cases to unambiguously ascertain the structures.
We tried this protocol with an aliphatic substituted 1,5-diketone system (3p) as well in the hope of getting fully substituted thiophene. The expected thiophene 4s was not obtained under the optimized conditions. This could be due to the poor regioselectivity among the three acidic centres for the initial anion generation (Scheme 4).
Conclusion
In conclusion, we have developed a rapid and experimentally convenient greener tool for the synthesis of thiophene derivatives from various 1,5-diketones with elemental sulfur in excellent yield. Participation of elemental sulfur in the thiophene ring formation similar to Gewald thiophene synthesis is another highlight of this method.Experimental
General remarks
A CEM Discover microwave synthesizer (Model no. 908010) operating at 180/264 V and 50/60 Hz with microwave power maximum level of 300 W and microwave frequency of 2455 MHz was employed for the microwave-assisted experiments. Nuclear Magnetic Resonance (1H and 13C NMR) spectra were recorded on a 300 MHz spectrometer in CDCl3 using TMS as an internal standard. Chemical shifts are reported in parts per million (δ), coupling constants (J values) are reported in Hertz (Hz) and spin multiplicities are indicated by the following symbols: s (singlet), d (doublet), t (triplet), p (pentad), m (multiplet). 13C NMR spectra were routinely run with broadband decoupling. Pre coated silica gel on aluminium plates (Merck) were used for TLC analysis with a mixture of petroleum ether (60–80 °C) and ethyl acetate as eluent. Elemental analyses were performed on a Perkin Elmer 2400 Series II Elemental CHNS analyzer. Spectra were recorded in LCQ Fleet spectrometer, Thermo Fisher Instruments Limited, US. Electrospray ionization spectrometry (ESI-MS) analysis was performed in the positive ion and negative ion mode on a liquid chromatography ion trap.General procedure for the synthesis of 1,3,5-triarylpentan-1,5-diones (3)
A mixture of chalcone 1 (1.0 equiv.), aryl methyl ketone 2 (1.0 equiv.) and powdered sodium hydroxide (2.0 equiv.) was crushed together for twenty minutes using a pestle and mortar. The mixture got solidified and the completion of the reaction was monitored by TLC chromatography using petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate mixture (4
ethyl acetate mixture (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. After the completion of the reaction, the reaction mass was washed with water to remove the sodium hydroxide to give the corresponding 1,3,5-triarylpentan-1,5-diones. The product was further purified by recrystallization from ethanol to give colorless crystals.
1) as eluent. After the completion of the reaction, the reaction mass was washed with water to remove the sodium hydroxide to give the corresponding 1,3,5-triarylpentan-1,5-diones. The product was further purified by recrystallization from ethanol to give colorless crystals.
General procedure for the synthesis of 2-aroyl thiophene (4)
A mixture of 1,3,5-triarylpentane-1,5-dione 3 (1.0 equiv.), morpholine (2.0 equiv.) and elemental sulfur (1.1 equiv.) was taken in a 10 mL quartz vial and placed in the microwave oven. The vial was sealed and subjected to microwave irradiation at 110 °C for 5 min. The reaction was monitored by TLC chromatography using petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate mixture (4
ethyl acetate mixture (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. After the completion of the reaction, the reaction was cooled to room temperature and ice cooled water was added. The precipitate obtained was filtered, dried in vacuum and recrystallized from ethanol to afford 4.
1) as eluent. After the completion of the reaction, the reaction was cooled to room temperature and ice cooled water was added. The precipitate obtained was filtered, dried in vacuum and recrystallized from ethanol to afford 4.
Acknowledgements
We thank the University Grant commission for financial support under the scheme of Minor Research project F. No MRP-5176/14 (SERO/UGC) and K. R gratefully acknowledges the award of a Junior Research Fellowship and financial support from CSIR, New Delhi Grant No. 02 (0061)/12/EMR-II. The authors thank DST, New Delhi for assistance under the IRHPA program for the NMR facility.Notes and references
- (a) V. G. Kharchenko, N. V. Pchelintseva, L. I. Markova and O. V. Fedotova, Chem. Heterocycl. Compd., 2000, 36, 1007 CrossRef CAS; (b) V. G. Kharchenko, L. I. Markova, O. V. Fedotova and N. V. Pchelintseva, Chem. Heterocycl. Compd., 2003, 39, 1121 CrossRef CAS.
- (a) R. K. Russell and J. B. Press, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees, E. W. F. Scriven and A. Padwa, Pergamon, New York, 1996, vol. 2, p. 679729 Search PubMed; (b) C. Wu, E. R. Decker, N. Blok, H. Bui, T. J. You, J. Wang, A. R. Bourgoyne, V. Knowles, K. L. Berens, G. W. Holland, T. A. Brock and R. A. F. Dixon, J. Med. Chem., 2004, 47, 1969 CrossRef CAS PubMed.
- (a) K. Dore, S. Dubus, H. A. Ho, I. Levesque, M. Brunette, G. Corbeil, M. Boissinot, G. Boivin, M. G. Bergeron, D. Boudreau and M. Leclerc, J. Am. Chem. Soc., 2004, 126, 4240 CrossRef CAS PubMed; (b) A. D. Pillai, P. D. Rathod, F. P. Xavier, K. K. Vasu, H. Padh and V. Sudarsanam, Bioorg. Med. Chem., 2004, 12, 4667 CrossRef CAS PubMed; (c) A. C. Ramos, R. Pelaez, J. L. Lopez, E. Caballero, M. Medarde and A. San Feliciano, Tetrahedron, 2001, 57, 3963 CrossRef CAS.
- C. Wu, E. R. Decker, N. Blok, H. Bui, T. J. You, J. Wang, A. R. Bourgoyne, V. Knowles, K. L. Berens, G. W. Holland, T. A. Brock and R. A. F. Dixon, J. Med. Chem., 2004, 47, 1969 CrossRef CAS PubMed.
- K. Doré, S. Dubus, H. A. Ho, I. Lévesque, M. Brunette, G. Corbeil, M. Boissinot, G. Boivin, M. G. Bergeron, D. Boudreau and M. Leclerc, J. Am. Chem. Soc., 2004, 126, 4240 CrossRef PubMed.
- C. Rost, S. Karg, W. Riess, M. A. Loi, M. Murgia and M. Muccini, Appl. Phys. Lett., 2004, 85, 1613 CrossRef CAS PubMed.
- D. M. Vriezema, J. Hoogboom, K. Velonia, K. Takazawa, P. C. M. Christianen, J. C. Maan, A. E. Rowan and R. J. M. Nolte, Angew. Chem., Int. Ed., 2003, 42, 772 CrossRef CAS PubMed.
- H. Yu, A. E. Pullen, M. G. Büschel and T. M. Swager, Angew. Chem., Int. Ed., 2004, 43, 3700 CrossRef CAS PubMed.
- (a) M. S. Yen and I. J. Wang, Dyes Pigm., 2004, 61, 243 CrossRef CAS PubMed; (b) H. Hartmann, P. Gerstner and D. Rohde, Org. Lett., 2001, 39, 1673 CrossRef PubMed.
- (a) H. Wynberg and H. J. Kooreman, J. Am. Chem. Soc., 1965, 87, 1739 CrossRef CAS; (b) G. Ravindran, N. Paul, S. Muthusubramanian and S. Perumal, J. Sulfur Chem., 2008, 29, 575 CrossRef CAS PubMed; (c) Y. Miyahara, E. Nishimura and T. Sugimura, J. Org. Chem., 2008, 73, 1783 CrossRef CAS PubMed.
- (a) For general methods, see:S. Gronowitz, in The Chemistry of Heterocyclic Compounds, Thiophene and its Derivatives, ed. S. Gronowitz, Wiley, New York, 1985, vol. 44, Part 1, p. 1 Search PubMed (b) R. A. Jones and P. U. Civcir, Tetrahedron, 1997, 53, 11529 CrossRef CAS; (c) F. Freeman, M. Y. Lee, H. Lu, X. Wang and E. Rodriguez, J. Org. Chem., 1994, 59, 3695 CrossRef CAS.
- (a) D. Obrecht, F. Gerber, D. Sprenger and T. Masquelin, Helv. Chim. Acta, 1997, 80, 531 CrossRef CAS PubMed; (b) C. E. Hewton, M. C. Kimber and D. K. Taylor, Tetrahedron Lett., 2002, 43, 3199 CrossRef CAS; (c) B. I. Drevko, E. V. Suchkova, G. A. Baranchikova and V. G. Mandych, Russ. Chem. Bull., 2006, 55, 1867 CrossRef CAS; (d) G. C. Nandi, S. Samai and M. S. Singh, J. Org. Chem., 2011, 76, 8009 CrossRef CAS PubMed; (e) H. Jiang, W. Zeng, Y. Li, W. Wu, L. Huang and W. Fu, J. Org. Chem., 2012, 77, 5179 CrossRef CAS PubMed.
- (a) T. Okazawa, T. Satoh, M. Miura and M. Nomura, J. Am. Chem. Soc., 2002, 124, 5286 CrossRef CAS PubMed; (b) I. Nakamura, T. Sato and Y. Yamamoto, Angew. Chem., Int. Ed., 2006, 45, 4473 CrossRef CAS PubMed; (c) W. You, X. Yan, Q. Liao and C. Xi, Org. Lett., 2010, 12, 3930 CrossRef CAS PubMed; (d) V. Guilarte, M. A. F. Rodríguez, P. G. García, E. Hernando and R. Sanz, Org. Lett., 2011, 13, 5100 CrossRef CAS PubMed; (e) B. Gabriele, R. Mancuso, L. Veltri, V. Maltese and G. Salerno, J. Org. Chem., 2012, 77, 9905 CrossRef CAS PubMed.
- (a) A. Fayol and J. Zhu, Angew. Chem., 2002, 114, 3785 CrossRef; (b) P. Janvier, X. Sun, H. Bienayme and J. Zhu, J. Am. Chem. Soc., 2002, 124, 2561 CrossRef PubMed; (c) P. Fontaine, G. Masson and J. Zhu, Org. Lett., 2009, 11, 1555 CrossRef CAS PubMed; (d) T. Piou, L. Neuville and J. Zhu, Org. Lett., 2012, 14, 3760 CrossRef CAS PubMed; (e) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMed.
- (a) N. Paul and S. Muthusubramanian, Tetrahedron Lett., 2011, 52, 3743 CrossRef CAS PubMed; (b) S. Chitra, N. Paul, S. Muthusubramanian and P. Manisankar, RSC Adv., 2012, 2, 1432 RSC; (c) M. Nagaraj, M. Boominathan, S. Muthusubramanian and N. Bhuvanesh, Org. Biomol. Chem., 2011, 9, 4642 RSC; (d) N. Paul, S. Muthusubramanian and N. Bhuvanesh, New J. Chem., 2011, 35, 2607 RSC; (e) K. Rajaguru, R. Suresh, A. Mariappan, S. Muthusubramanian and N. Bhuvanesh, Org. Lett., 2014, 16, 744 CrossRef CAS PubMed; (f) A. Mariappan, K. Rajaguru, S. Muthusubramanian and N. Bhuvanesh, Tetrahedron Lett., 2015, 56, 338 CrossRef CAS PubMed; (g) N. Paul, R. Sathishkumar, C. Anuba and S. Muthusubramanian, RSC Adv., 2013, 3, 7445 RSC.
- (a) R. W. Sabnis, Sulfur Rep., 1994, 16, 1 CrossRef CAS PubMed; (b) R. Mayer and K. Gewald, Angew. Chem., Int. Ed. Engl., 1967, 6, 294 CrossRef CAS PubMed; (c) K. Gewald, E. Schinke and H. Bottcher, Chem. Ber., 1966, 99, 94 CrossRef CAS PubMed; (d) K. Gewald, G. Neumann and H. Z. Bottcher, J. Chem., 2010, 6, 261 Search PubMed; (e) K. Gewald and E. Schinke, Chem. Ber., 1966, 99, 2712 CrossRef PubMed; (f) I. L. Pinto, R. L. Jarvest and H. T. Serafinowska, Tetrahedron Lett., 2000, 41, 1597 CrossRef CAS; (g) M. Mittelbach and H. Junek, Liebigs Ann. Chem., 1986, 533 CrossRef CAS PubMed; (h) M. Zhang and R. W. Harper, Bioorg. Med. Chem. Lett., 1997, 7, 1629 CrossRef CAS; (i) M. Sridhar, R. M. Rao, N. H. K. Baba and R. M. Kumbhare, Tetrahedron Lett., 2007, 48, 3171 CrossRef CAS PubMed; (j) G. Hallas and J. H. Choi, Dyes Pigm., 1999, 40, 99 CrossRef CAS; (k) K. Balamurugan, S. Perumal, K. Reddy, P. Yogeeswari and D. Sriram, Tetrahedron Lett., 2009, 50, 6191 CrossRef CAS PubMed.
- (a) X. G. Huang, J. Liu, J. Ren, T. Wang, W. Chen and B. B. Zeng, Tetrahedron, 2011, 67, 6202 CrossRef CAS PubMed; (b) D. M. Barnes, A. R. Haight, T. Hameury, M. A. McLaughlin, J. Mei, J. S. Tedrow and J. D. Riva Toma, Tetrahedron, 2006, 62, 11311 CrossRef CAS PubMed; (c) V. P. Litvinov, Russ. Chem. Rev., 1999, 68, 737 CrossRef CAS PubMed; (d) V. P. Litvinov, Russ. Chem. Rev., 1999, 68, 39 CrossRef CAS PubMed; (e) K. Wang, D. Kim and A. Domling, J. Comb. Chem., 2010, 12, 111 CrossRef CAS PubMed.
- X. Lei and X. Bai, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, 65, o514 CAS.
- (a) S. Tu, C. Li, G. Li, L. Cao, Q. Shao, D. Zhou, B. Jiang, J. Zhou and M. Xia, J. Comb. Chem., 2007, 9, 1144 CrossRef CAS PubMed; (b) S. Tu, S. Wu, S. Yan, W. Hao, X. Zhang, X. Cao, Z. Han, B. Jiang, F. Shi, M. Xia and J. Zhou, J. Comb. Chem., 2009, 11, 239 CrossRef CAS PubMed; (c) N. Ma, B. Jiang, G. Zhang, S. J. Tu, W. Weverb and G. Li, Green Chem., 2010, 12, 1357 RSC; (d) C. Cheng, B. Jiang, S. J. Tu and G. Li, Green Chem., 2011, 13, 2107 RSC.
- CCDC No. for compound 4a: 1062366.†.
- (a) T. Kanbara, Y. Kawai, K. Hasegawa, H. Morita and T. Yamamoto, J. Polym. Sci., Part A: Polym. Chem., 2003, 39, 3739 CrossRef PubMed; (b) Z. Puterová, A. Andicsová and D. Végh, Tetrahedron, 2008, 64, 11262 CrossRef PubMed; (c) I. V. Zavarzin, V. N. Yarovenko, A. V. Shirokov, N. G. Smirnova, A. A. Eśkov and M. M. Krayushkin, ARKIVOC, 2005, 13, 205 Search PubMed.
- J. Wang, X. Shi, X. Men and L. Zhao, Synth. Commun., 2003, 33, 2849 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1062366. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra17829k |
| This journal is © The Royal Society of Chemistry 2015 |