A double network strategy to improve epithelization of a poly(2-hydroxyethyl methacrylate) hydrogel for corneal repair application
Lei Wangb,
Conglie Lua,
Huihua Liub,
Sen Linab,
Kaihui Nanab,
Hao Chen*ab and
Lingli Li*ab
aSchool of Ophthalmology & Optometry and Eye Hospital, Wenzhou Medical University, Zhejiang Province, People's Republic of China. E-mail: Lingli_lee@yeah.net; Chenhao@mail.eye.ac.cn
bWenzhou Institute of Biomaterials and Engineering, Zhejiang Province, People's Republic of China
First published on 15th December 2015
Abstract
To enhance the epithelization of poly(2-hydroxyethyl methacrylate) hydrogels and provide a biocompatible alternative for keratoplasty, this paper presents a novel double network scaffold and its preparation methods, in which a cell-affinitive hydrogel was made by poly(2-hydroxyethyl methacrylate) and modified gelatin. Methacrylic anhydride modified gelatin was interpenetrated into the as-prepared poly-2-hydroxyethyl methacrylate to form a porous double network to facilitate cell behavior on the hydrogels. Physico-chemical measurements such as X-ray photoelectron spectroscopy, transparency, swelling ratio, scanning electron microscopy, thermal gravimetric analysis as well as dynamic mechanical analysis have been performed in order to correlate the material composition with the corresponding properties. The in vitro cell assays of the porous double networks revealed that cells remained viable and, depending on the composition, were able proliferate, as was demonstrated by the deposition of gelatin. All of these results demonstrated that these porous double networks possess more advantages compared with poly(2-hydroxyethyl methacrylate) alone and improved epithelization due to methacrylated gelatin. The obtained double network scaffold could act as an attractive material for corneal repair application.
1. Introduction
According to a recent report, about 4 million people worldwide suffer from corneal blindness.1 The most successful and widely accepted treatment for corneal blindness worldwide is to replace the blind cornea with a transparent and healthy donor cornea.2 However, the worldwide limited availability of suitable high quality corneal donor tissue, the short shelf-life of suitable corneas and the problem of their preservation, still remain the fundamental problems with corneal replacement. In addition, the increasing use of Laser-Assisted In Situ Keratomileusis (LASIK) surgery for refractive correction has reduced the availability of donor cornea.3,4To overcome the lack of donor tissues especially in developing countries, some promising cornea alternatives, such as artificial corneas or a keratoprosthesis (Kpro), have been developed in recent decades. In order to achieve an ideal keratoprosthesis, corneal tissue equivalent needs to be biocompatible, mechanically equivalent to natural cornea so that its flexible structure allows to take the shape of the eye and lay flat on the surface, have the ability for oxygen and nutrient transfer through the structure, and exhibit anterior surface features that allow epithelialization and survival of corneal epithelial cells.5 The best known keratoprosthesis, such as AlohCor,6 Dohlman-Doane7 and Osteo-Odonto,8 have shown encouraging outcomes. However, these implants are still only reserved for cases in which human donor transplants fail.9 One of the most important problems with these materials is the lack of a stable re-epithelialization of the implant surface. Indeed, long-term performance of such keratoprosthesis would be improved if the materials could support stable epithelial cells growth on their surface.10 It has also been argued that epithelial coverage can minimized the risk of infections by serving as a barrier to microbial contamination and debris.11 Several attempts have been made to modify the surface of hydrogels to enhance epithelialization with some success,12,13 but to date, a keratoprosthesis that supports surface epithelialization has not been fully developed in human patients.
Poly(hydroxyethyl methacrylate) (PHEMA) hydrogels, as the main component of most successful artificial cornea Alphacor Kpro, show good biocompatibility, they are transparent, nutrients permeable and possess enough mechanical strength, but the re-epithelization of their surfaces is still representing the main problem, leading to transplant failure.14 Sheardown et al.15 adopted peptides such as arginylglycylaspartic acid (RGD) and Tyr-Ile-Gly-Ser-Arg (YIGSR), a laminin-derived peptide, to modify the surface of PHEMA in order to promote cell coverage. Jianjiang Xu et al.16 used hyaluronic acid and cationized gelatin to improve the cell adhesion in the PHEMA porous skirt to produce a firm bond between the remaining corneal tissue and the implants. Several PHEMA composites have been obtained by either hydrophobic materials such as methyl methacrylate (MMA) via copolymerization17 or hydrophilic macromolecules like poly(ethylene glycol) (PEG) by interpenetrating network approach18 with the intent to reach the same biocompatibility of the human cornea. In recent years, considerable efforts have been made on the development of double network (DN) hydrogel with good mechanical strength and high biocompatibility.19–22 Thus, the DN concept should be applicable to develop hydrogels with biochemical properties using combinations of various biopolymers and natural macromolecules.
Inspired by the DN strategy, we present improvements to the cellular affinity of artificial cornea made by a DN formed with PHEMA and modified natural macromolecules. Thus, we developed a novel hydrogel material possessing biomechanical properties and biocompatibility, both representing the main features of the ideal artificial cornea. In particular, this hydrogel was a DN network consisting of PHEMA as first network and Gel-MA as second network. In this study we described the synthesis of a bio-affinitive, photo-curing and membrane-forming methacrylated gelatin (Gel-MA), which was interpenetrated into the PHEMA network to enhance its biocompatibility. The chemical composition, microscopic morphology, equilibrium water content, transparency, thermal stability and mechanical property were examined. Cell viability and re-epithelization behavior was also investigated in detail using human corneal epithelial cells.
2. Experimental
2.1 Materials
Type-A porcine skin gelatin (Bloom 100), 2-hydroxyethyl methacrylate (HEMA), triethylene glycol dimethacrylate (TEGDMA), azodiisobutyronitrile (AIBN), methacrylic anhydride (MA) and photo initiator Irgacure 2959 (I2959) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All the reagents used in this study were used as purchased without further purification. Milli-Q grade water (Millipore, Bedford, MA, USA) was used in the preparation of the solutions.2.2 Polymer synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and AIBN were dissolved in a mixed solution of water and acetone (volume ratio 1
1), and AIBN were dissolved in a mixed solution of water and acetone (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The homogeneous solution was poured into a plastic plate placed in a water bath at 50 °C for 24 h to allow free radical polymerization. Hydrogels of different solid contents were prepared and washed by Milli-Q water to remove the unreacted residual monomers.
1). The homogeneous solution was poured into a plastic plate placed in a water bath at 50 °C for 24 h to allow free radical polymerization. Hydrogels of different solid contents were prepared and washed by Milli-Q water to remove the unreacted residual monomers.2.3 1H NMR
1H NMR was recorded on a Bruker NMR (Advanced III, 600 MHz, Bruker, Germany) using D2O as solvent. The degree of methacrylation was quantified by the Habeeb method22,23 and 1H NMR method previously described19 for methacrylate modified collagen.242.4 PDN fabrication process
The as-prepared PHEMA membranes were presoaked in two solutions containing different concentrations of Gel-MA (50 mg mL−1, samples named as PDN-50; 100 mg mL−1, named as PDN-100) and 0.5 wt% solution of the photo initiator I2959 at 37 °C for 24 h in the dark. Samples were subjected to irradiation using UV Light-curing Unit (YDL, HANGZHOU YINYA NEW MATERIALS CO., LTD). All the samples were placed at a distance of 3 cm from the radiation source (light intensity: 90 mW cm−2) and exposed for 90 s. The resulting membranes were immersed in warm double-distilled water (37 °C) to remove unreacted Gel-MA and residual initiators.2.5 X-ray photoelectron spectroscope (XPS)
The detailed chemical structures of the PDN hydrogels were further confirmed by X-ray Photoelectron Spectroscope (XPS, AXIS ULTRA DLD SHIMADZU, Japan). XPS was recorded using a thermo spectrometer with a mono-chromatized AlKα X-ray source (1486.6 eV photons). Surface elemental compositions were determined from the peak-area ratios, after correction with the experimentally determined sensitivity factors, and were reliable to ±5%. The elemental sensitivity factors were determined using stable binary compounds of well-established stoichiometry.2.6 Scanning electron microscopy
The morphology of the hydrogels (Gel-MA, PDN hydrogels and pure PHEMA) was observed using a scanning electron microscopy (Nova NanoSEM200, FEI, USA). Lyophilized hydrogel samples (Φ 6 mm) were sputter-coated with gold for 60 s before the observation.2.7 Optical transmittance measurements
The optical transmittance of the hydrogels was measured using the ultraviolet-visible spectrophotometer (U-4100 Spectrophotometer Hitachi, Japan) operating in a spectral range of 380–780 nm with a scan speed of 300 nm min−1 and sampling interval of 1 nm.2.8 Equilibrium water content
The equilibrium water content (EWC) of each sample was determined by immersing them in PBS at 34 °C (physiological temperature of the cornea).25 The equilibrium water content was defined as follows:26where Weq is the weight of the swollen hydrogel after reaching the equilibrium state and Wd is its initial dry weight.
2.9 Thermal analysis
The thermogravimetric analysis of PDN hydrogels was obtained using a Perkin-Elmer TGA7 thermo balance. The experiments were performed under nitrogen flux of 90 mL min−1, at a heating rate of 10 °C min−1 from 0 to 700 °C, using samples of approximately 10 mg.2.10 Dynamic mechanical analysis
Dynamic mechanical analysis was performed using a DMA/SDTA861e DMA (Mettler Toledo, GER), equipped with a tensile-testing mode in a controlled environment at 37 °C. PDN hydrogels were cut into strips (length = 10 mm, width = 5 mm, thickness = 1 mm) in their equilibrium swollen state. DMA spectra were obtained during a frequency scan that varied between 0.1 and 10 Hz.2.11 Cell culture
Human Corneal Epithelial Cells (HCEC) were cultured in Dulbecco's Modified Eagle Medium nutrient mixture F-12 (DMEM/F-12, Gibco, Life Technologies, USA), supplemented with 10% (v/v) fetal bovine serum (Gibco, Life Technologies, USA), 1% (v/v) penicillin–streptomycin (Gibco, Life Technologies, USA), 5 μg mL−1 human recombinant insulin (Sigma-Aldrich, USA) in a humidified incubator at 37 °C and 5% CO2. The media were changed every two days. Trypsin–EDTA 0.05% was used for cell passage when HCEC reached 90% confluence.2.12 Cell viability assay
The cytotoxicity of the PDN hydrogels was evaluated as follows. All the sterilized samples were placed into wells of a 96-well polystyrene cell culture plate and soaked overnight in two changes of culture media, then rinsed with phosphate buffer solution (PBS) to remove soluble impurities. Cells were seeded at the concentration of 1 × 103 per each well. At specific time points, the cells were stained with LIVE/DEAD® Viability/Cytotoxicity Kit (Life Technologies, USA), which is cleaved by metabolically active cells to yield a green fluorescent product, while dead cells produce a red fluorescent product. Samples treated with 75% ethanol for 10 minutes were used as negative controls. Samples were incubated in live/dead assay dye solution (0.25 μL calcein-AM dye and 1.0 μL ethidium homodimer-1 dye in 0.5 mL PBS) for 30 min. After incubation, the samples were rinsed with PBS for 3 times and observed under a Nikon Eclipse Ti microscope (Transfer ManNK2, Nikon ECLIPSE Ti, Japan). All experiments were performed in triplicate. In addition, cell viability through mitochondrial activity was measured by a CCK-8 assay. HCEC were seeded on hydrogels as described above and incubated at specific time intervals. Cells cultured in the same cell culture medium with 5% DMSO were used as negative controls. At each time point (1 day, 3 days, 5 days and 7 days), the samples were washed with PBS and incubated with CCK-8 for 2 h at 37 °C. These procedures were carried out in the dark. The OD value was measured using an ELISA plate reader (WD-2102A, BeiJing Liuyi instrument plant) and the cell viability (%) was calculated using the following equation:27| Cell viability (%) = ODtest/ODcontrol × 100% |
2.13 Statistical analysis
All data were expressed as the mean ± SD. Student's t-test was used to compare the mean values from two groups. The value of p < 0.05 was considered statistically significant and marked with an asterisk in the figures. All analyses were performed using Origin 8.0.3. Results and discussion
3.1 Synthesis and characterization of Gel-MA
Gelatin is a versatile biomedical macromolecule containing many amino and carboxylic groups. To endow it with UV-crosslinking ability, gelatin was modified by adding methacrylate groups to the amino groups of gelatin. Fig. 1 shows the scheme of facile synthesis of UV-crosslinkable gelatin without using any coupling agents or catalysts; the incorporated methacrylate groups conferred the UV-crosslinking ability to the gelatin. The chemical structure of methacrylated gelatin was confirmed by 1H NMR spectrum, which clearly showed the signals of vinyl protons at 5.34 and 5.60 ppm. The weak peaks at 5.44 and 5.62 are attributed to existence of the unreacted MA. No chemical shift appeared at 5.2–6.0 ppm in the native gelatin NMR spectrum, as confirmed by another work,28 and only vinyl proton are the one showing chemical shift signals at 5.5–6.0. 1H NMR spectra result corresponded with the schematic structure of Gel-MA, which was due to the acylation between amino groups and MA. The degree of methacrylation calculated from 1H NMR was 25.10%.3.2 Fabrication of hydrogels
PHEMA-based materials have been widely used as hydrogels to develop many ophthalmic devices such as soft contact and intraocular lenses, artificial cornea and drug delivery systems.29–31 To simulate the micro-architecture and composition of cornea, we fabricated PDN hydrogels by physically incorporating and covalently bounding a protein into a synthetic polymer network (Fig. 2). First, a PHEMA network was created by free-radical polymerization with the help of cross-linking agent TEGDMA (Fig. 2a). The second network was formed by immersion of PHEMA network into the Gel-MA solution (Fig. 2c). Compared to the Gel-MA single polymer network (Fig. 2b and e), the double polymer network was topologically more interlocked (Fig. 2f). The synthesized PDN hydrogels were optically clear, transparent and could be easily handled with forceps.3.3 Synthesis of the porous double network (PDN)
Table 1 shows the elemental compositions of Gel-MA, PHEMA, PDN-50 and PDN-100 calculated from XPS wide spectrum results. The N content of the PDN-100 (atomic concentration 11.96%) is higher than that of PDN-50 (8.83%), indicating that the increased concentration of Gel-MA resulted in a more Gel-MA interpenetration into the PHEMA structure, thus exhibiting stronger signals. The C 1s XPS signals of PHEMA, Gel-MA, PDN-50 and PDN-100 are shown in Fig. 3. PHEMA peak-fitted XPS scan C 1s spectrum showed three components representing the carbon atoms in different functional groups: (1) aliphatic hydrocarbon (C–C/C–H, at a binding energy of 284.60 eV), (2) hydroxyl or ester bond (C–O at 286.20 eV), and (3) carboxyl carbon (–COO at 288.45 eV). Gel-MA C 1s spectrum also showed three different functional groups of carbon atoms: (1) aliphatic hydrocarbon (C–C/C–H, at a binding energy of 284.60 eV), (2) amide bond (C–N at 285.92 eV), and (3) –CONH at 287.67 eV. The C 1s spectra of both the PDN showed peaks corresponding to four functional groups: (1) aliphatic hydrocarbon (C–C/C–H, at a binding energy of 284.60 eV), (2) amide bond or ester bond (C–N/C–O, at 286.05 eV, a bit shifted because of the inter-molecular hydrogen-bond interaction), (3) amide bond (–CONH at 287.70 eV), (4) ester bond (–COO at 288.95 eV).| Samples | Atomic concentration (%) | ||
|---|---|---|---|
| Elements | |||
| O | C | N | |
| Gel-MA | 22.56 | 64.88 | 12.56 |
| HEMA | 29.65 | 70.35 | 0 |
| PDN-50 | 23.37 | 67.80 | 8.83 |
| PDN-100 | 19.96 | 68.08 | 11.96 |
3.4 SEM
Typical SEM images showing the morphology of the hydrogels surface are shown in Fig. 4. The images confirmed the presence of different porous structure in the synthetized PDN hydrogels. The pore sizes of the PDN-50 (Fig. 4C) and PDN-100 (Fig. 4D) are about 100 μm. These pores were permeable to water and nutrients, allowing their efficient uptake. The inner structure of the PHEMA hydrogels shown in Fig. 4A was compact and dense, while the outer structures of the PDN-50 and PDN-100 in the Fig. 4C and D showed an interconnected highly porous network due to the involvement of Gel-MA, as demonstrated by the feature of Gel-MA in Fig. 4B. The change in morphology provided some evidence to the swelling behaviors of the hydrogels. | ||
| Fig. 4 SEM images of surface: (A) single network of PHEMA; (B) single network of Gel-MA; (C) PDN-50; (D) PDN-100. | ||
3.5 Equilibrium water content
The EWC of the PDN hydrogels was investigated in PBS. The effect of chemical composition on EWC is depicted in Fig. 5. The main factor affecting the EWC of PDN hydrogels was its chemical constitution. PHEMA possessed a low swelling ratio, causing some problems during its use as previously reported,32,33 while the single Gel-MA was rapidly swollen, being difficult to weigh it. As shown in Fig. 5, compared to pure PHEMA (EWC: 30.36%), the PDNs' EWC was increased as expected (PDN-100: 74.4%, similar to human cornea;5 PDN-50: 52.3%) and increased with increased concentrations of Gel-MA. Thus, it may be speculated that higher water content leads to an increased permeability to nutrients such as proteins and saccharides, which is an extremely important factor for the re-growth of the epithelium.3.6 Optical transparency
Transparency measurements of PDN hydrogels are shown in Fig. 6. The transmission curve of the hydrogels was significantly increased in the visible region (380 to 780 nm). The films of pure PHEMA were almost non-transparent with a transmittance of 27%. However, with the modification induced by Gel-MA, the transmittance of PDN-100 (82%) was slightly higher than PDN-50 (74%), which were both quite closer to that of human cornea reported by Rafat (around 60%).343.7 Thermal analysis
Since the TGA provides information regarding the thermal decomposition behavior, the thermal stability and thermal decomposition of PDN hydrogels were investigated using TGA and the test results are shown in Fig. 7. The TGA curves obtained by plotting the percentage of weight loss against temperature indicated that Gel-MA and PHEMA were stable up to a temperature of 249 °C and 195 °C respectively. The maximum degradation rate of samples move to higher temperature as the content of Gel-MA increase in the PDN structure, which could be due to entanglements formation between the different macromolecular chains that can be stabilized by hydrogen bonds.The thermal stability and thermal degradation phenomena of polymeric materials are related to the microstructure, especially the intra- and inter-molecular interactions between the polymeric chains. They reflects a good interconnection between both types of polymeric chains in the membrane, which could be due to the crosslinking reaction but also to the entanglements between the different macromolecular chains stabilized by hydrogen bonds. However, for both PDN-50 and PDN-100 sample, two maxima were presented, indicating that the interconnection between Gel-MA and PHEMA macromolecules is not so strong.
3.8 Dynamic mechanical analysis
To analyze the mechanical properties of the PDN networks, hydrogels were subjected to dynamics consecutive frequency sweeps ranging from 0.1 to 10 Hz, at a temperature of 37 °C. Storage or elastic modulus (G′) was measured as a function of the frequency. The data in Fig. 8 showed that the storage modulus of all the hydrogels increased with increasing frequency, although the increased profiles were different among the different hydrogels. The modulus values of PDN hydrogels decreased with the incorporation of the second network. This decrease may be due to the brittle nature of Gel-MA that affect the mechanical property of PDN hydrogels. However, all the modulus of the as-prepared PDN hydrogels are similar to the Young's modulus of human cornea, which have varied from 0.15–57 MPa.35–42 As for Gel-MA hydrogel alone, it is too weak to be tested. | ||
| Fig. 8 Storage modulus (G′) of hydrogels according to the applied frequency measured at 37 °C, in a hydrated environment. | ||
3.9 In vitro cytocompatibility analysis
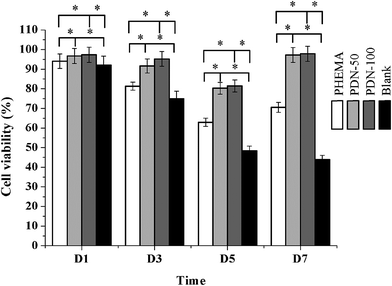
Since the cytocompatibility is a fundamental and crucial property for biomaterials to maintain normal cells survival, proliferation and differentiation, the in vitro cytocompatibility of PDNs were evaluated by CCK-8 assay using HCEC. After 1, 3, 5 and 7 days of culturing, the CCK-8 assay was performed to detect the potential cytotoxicity exerted by the hydrogels by analyzing the OD492 nm values. Fig. 9 shows the OD values of hydrogels on each time point. No statistically significant differences were observed in HCEC viability between PDN-50 and PDN-100 compared with the control group, although the average cell viability was lower than that of the control group. HCEC proliferation revealed that PDN-50 and PDN-100 had no negative effect on cell growth and proliferation, and no cytotoxic compounds had been released by any of the two hydrogels. In addition, statistically significant differences in the cell viability were observed between PHEMA and PDN hydrogels. The OD values of PDN films increased with increasing culture time, suggesting that HCEC cells could adhere and proliferate on PDN-50 and PND-100 compared to PHEMA, results that were confirmed by Live/Dead assay. Fig. 10 shows that the HCEC cells were uniformly spreading on the PDN gels and grew with normal morphology, while neither living cell nor dead cell could be seen on pure PHEMA. Viable cells were visible by LIVE/DEAD assay for up to 7 days with no cells on the PDN hydrogels showing red fluorescence, suggesting that the incorporation of Gel-MA into the PHEMA could enhance its in vitro biocompatibility and epithelization. This result may be due to several advantages of Gel-MA, thanks to its natural properties, which allowed a cell-responsive behavior, including the presence of cell-adhesion sites and proteolytic biodegradability.43,44 All of the results made clear that the porous double network structure reached the essential condition for improving epithelization not only because offered enough space for cell, but also provided a higher permeability for a sufficient supply of oxygen and nutrients from the medium important for HCEC survival and proliferation.4. Conclusions
In this study, novel porous double network hydrogels were successfully fabricated. The swelling and transparency characteristics of the PDN hydrogels could be adjusted by changing the concentration of Gel-MA to reach the requirements of human cornea. In vitro cytotoxicity indicated that the PDN hydrogels were non-toxic to HCEC after 7 days of incubation. Furthermore, LIVE/DEAD assay also showed that the adhered HCECs can grow and proliferate on the PDN hydrogels with a normal morphology compared to PHEMA. In addition, the mechanical property of PHEMA was not significantly affected by the incorporation of Gel-MA network. In summary, the crosslinking of excellent natural protein within a synthetic matrix such as PHEMA, created a stable scaffold supporting corneal epithelial cell attachment and proliferation, which could represent a potential candidate for corneal repair and regeneration.Acknowledgements
This work has been partly supported by The International Scientific and Technological Cooperation Project (No. 2012DFB30020), Zhejiang Provincial Natural Science Foundation (LQ14C100003), Wenzhou Municipal Public Welfare Foundation (Y20140701) and Wenzhou Science and Technology Foundation (2013S0405). The authors would like to thank Dr Timothy Hughes (CSIRO) for kindly providing help and useful suggestions on preparation of materials.References
- D. Mariotti SP, Br. J. Ophthalmol., 2012, 96, 614 CrossRef PubMed.
- J. Wu, Y. Du, M. M. Mann, E. Yang, J. L. Funderburgh and W. R. Wagner, Tissue Eng., Part A, 2013, 19, 2063 CrossRef CAS PubMed.
- A. Z. Crawford, D. V. Patel and C. McGhee, Oman Journal of Ophthalmology, 2013, 6, S12 Search PubMed.
- N. Polisetti, M. M. Islam and M. Griffith, Methods Mol. Biol., 2013, 1014, 45 CAS.
- T. V. Chirila, C. R. Hicks, P. D. Dalton, S. Vijayasekaran, X. Lou, Y. Hong, A. B. Clayton, B. W. Ziegelaar, J. H. Fitton, S. Platten, G. J. Crawford and I. J. Constable, Prog. Polym. Sci., 1998, 23, 447 CrossRef CAS.
- P. V. Andel, P. Cuperus, M. Kolenbrander, D. Singh and J. Worst, Transactions Third World Biomaterials Congress, Kyoto, Japan, 1988, p. 542 Search PubMed.
- J. M. Legeais, G. Renard, J. M. Parel, O. Serdarevic, M. Mei-Mui and Y. Pouliquen, Exp. Eye Res., 1994, 58, 41 CrossRef CAS PubMed.
- H. Cardona, Am. J. Ophthalmol., 1964, 58, 247 CrossRef CAS PubMed.
- C. R. Hicks, T. V. Chirila, A. B. Clayton, J. H. Fitton, S. Vijayasekaran and P. D. Dalton, et al., Clinical results of implantation of the Chirila keratoprosthesis in rabbits, Br. J. Ophthalmol., 1998, 82, 18–25 CrossRef CAS PubMed.
- C. R. Hicks, G. J. Crawford, X. Lou, D. T. Tan, G. R. Snibson, G. Sutton, N. Downie, L. Wener, T. V. Chirila and I. J. Constable, Eye, 2003, 17, 385 CrossRef CAS PubMed.
- D. Duan, B. J. Klenkler and H. Sheardown, Expert Rev. Med. Devices, 2006, 3, 59 CrossRef CAS PubMed.
- X. Y. Wu, A. Cornell-Bell, T. A. Davies, E. R. Simons and V. Trinkaus-Randall, Invest. Ophthalmol. Visual Sci., 1994, 35, 878 CAS.
- X. Jun, J. Sun, J. Hong, W. Wang, A. Wei, Q. Le and J. Xu, Mater. Sci. Eng., 2015, 50, 274 CrossRef PubMed.
- Y. Hou, C. Chen, K. Liu, Y. Tu, L. Zhang and Y. Li, RSC Adv., 2015, 5, 24023 RSC.
- K. Merrett, C. M. Griffith, Y. Deslandes, G. Pleizier and H. Sheardown, J. Biomater. Sci., Polym. Ed., 2001, 12, 647 CrossRef CAS PubMed.
- J. Xiang, J. Sun, J. Hong, W. Wang, A. Wei, Q. Le and J. Xu, Mater. Sci. Eng., C, 2015, 50, 274 CrossRef CAS PubMed.
- Y. Shi, H. Lv, Y. Fu, Q. Lu, J. Zhong, D. Ma, Y. Huang and W. Xue, Biomed. Mater., 2013, 8(5), 5507 Search PubMed.
- S. Park, S. H. Nam and W. G. Koh, J. Appl. Polym. Sci., 2012, 123, 637 CrossRef CAS.
- T. C. Suekama, J. Hu, T. Kurokawa, J. P. Gong and S. H. Gehrke, ACS Macro Lett., 2013, 2, 137 CrossRef CAS.
- L. Cai, R. E. Dewi and S. C. Heilshorn, Adv. Funct. Mater., 2015, 25, 1344 CrossRef CAS PubMed.
- Q. Chen, H. Chen, L. Zhu and J. Zheng, J. Mater. Chem. B, 2015, 3, 3654 RSC.
- J. A. Benton, C. A. DeForest, V. Vivekanandan and K. S. Anseth, Tissue Eng., Part A, 2009, 15(11), 3221 CrossRef CAS PubMed.
- A. F. Habeeb, Anal. Biochem., 1966, 14(3), 328 CrossRef CAS PubMed.
- W. T. Brinkman, K. Nagapudi, B. S. Thomas and E. L. Chaikof, Biomacromolecules, 2003, 4(4), 890 CrossRef CAS PubMed.
- T. Moller-Pederson, U. Hartmann, H. J. Moller, N. Ehlers and K. Engelmann, Br. J. Ophthalmol., 2001, 85, 1075 CrossRef.
- M. D. M. Evans, H. Chaouk, J. S. Wilkie, B. A. Dalton, S. Taylor, R. Z. Xie, T. C. Hughes, G. Johnson, G. A. McFarland, H. H. Griesser, J. G. Steele, G. F. Meijs, D. F. Sweeney and K. M. McLean, Biomaterials, 2011, 32, 8870 CrossRef CAS PubMed.
- C. Shen, Y. Han, B. Wang, J. Tang, H. Chen and Q. Lin, RSC Adv., 2015, 5, 53782 RSC.
- B. F. Pierce, G. Tronci, M. Rößle, A. T. Neffe, F. Jung and A. Lendlein, Macromol. Biosci., 2012, 12, 484 CrossRef CAS PubMed.
- S. Garty, R. Shirakawa, A. Warsen, E. M. Anderson, M. L. Noble, J. D. Bryers, B. D. Ratner and T. T. Shen, Invest. Ophthalmol. Visual Sci., 2011, 52(9), 6109 CAS.
- N. A. Peppas and W. H. M. Yang, Contact Lens and Anterior Eye, 1981, 7, 300 CAS.
- C. Lu, R. B. Yoganathan, M. Kociolek and C. Allen, J. Pharm. Sci., 2012, 102(2), 623 Search PubMed.
- S. Vijayasekaran, T. V. Chirila, T. A. Robertson, X. Lou, J. H. Fitton, C. R. Hicks and I. J. Constable, J. Biomater. Sci., Polym. Ed., 2000, 1, 599 CrossRef.
- C. R. Hicks, L. Werner, S. Vijayasekaran, N. Mamalis and D. J. Apple, Cornea, 2005, 24, 933 CrossRef CAS PubMed.
- M. Rafat, F. Li, P. Fagerholm, N. S. Lagali, M. A. Watsky, R. Munger, T. Matsuura and M. Griffith, Biomaterials, 2008, 29, 3960 CrossRef CAS PubMed.
- H. Wang, P. I. Prendiville, P. J. McDonnell and W. V. Chang, J. Med. Biomech., 1996, 29, 1633 CrossRef CAS.
- G. Wlooensak, E. Spoerl and T. Seller, Am. J. Ophthalmol., 2003, 135, 620 CrossRef.
- A. Elshelkh, D. Wang and D. Pye, J. Cataract Refractive Surg., 2007, 23, 808 Search PubMed.
- T. T. Andreassen, A. H. Simonsen and H. Oxlund, Exp. Eye Res., 1980, 31, 435 CrossRef CAS PubMed.
- I. S. Nash, P. R. Greene and C. S. Foster, Exp. Eye Res., 1982, 35, 413 CrossRef CAS PubMed.
- C. Edmund, Acta Ophthalmol., Suppl., 1989, 193, 9 Search PubMed.
- S. Woo, A. Kobayashi, C. Lawrence and W. Schlegel, Am. J. Biomed. Eng, 1972, 1, 87 CAS.
- D. A. Hoeltzel, P. Altman, K. Buzard and K. Choe, J. Biomech. Eng., 1992, 114, 202 CrossRef CAS PubMed.
- W. J. Nichol, T. S. Koshy, H. Bae, M. C. Hwang, S. Yamanlar and A. Khademhosseini, Biomaterials, 2010, 31, 5536 CrossRef PubMed.
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |