Synthesis and fluorescence properties of a boron-dipyrrin functionalized perylenediimide derivative†
Huan-ren Chenga,
Ying Qian*a,
Yang Yangb and
Hui-ling Liuc
aSchool of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China. E-mail: yingqian@seu.edu.cn
bInstitute of Chemistry, Chineses Academy of Sciences, China
cNational Center for Nanoscience and Technology, China
First published on 6th October 2015
Abstract
In this paper, a novel dendritic chemosensor was designed and synthesized. Specific dual-channel fluorescence sensing for Hg2+ was investigated. The probe represented dual recognition sites and segmented detection behaviors, which can be deduced to the dethioacetalization reaction promoted by Hg2+ and the coordination reaction between Hg2+ ions and the nitrogen atoms in the triazole rings of the probe. The probe was proved to selectively recognize Hg2+ ions in aqueous solution and it showed a high selectivity, a low detection limit (12 nM), a short response time, and a significant fluorescence enhancement (at 610 nm) with a high fluorescence resonance energy transfer efficiency (99%) between the energy donor and the energy acceptor. Moreover, the energy levels of the energy donor–acceptor pairs calculated by DFT were used to illustrate the different FRET efficiencies of the probe and the probe with Hg2+.
1. Introduction
In recent years, fluorogenic research with fluorescence resonance energy transfer (FRET) systems has actively progressed in the region of supramolecular chemistry given its potentially practical benefits in the field of chemosensors toward targeted small molecules or ions.1–5 FRET play an important role in all kinds of light-harvesting systems, it arises from an excited-state energy interaction between two fluorophores in which an excited state donor transfers energy to a proximal ground state acceptor through long-range dipole–dipole interactions. Accordingly, the rate of energy transfer is strongly influenced by many factors, such as the extent of spectral overlap, the relative orientation of the transition dipoles, and the distance between the donor and acceptor.6–8 Therefore, it is not easy to design and synthesize a FRET-based chemosensor with high FRET efficiency.Mercury(II) ions accumulated in the human body often leads to severe diseases.9 Thus, obtaining effective methods for detecting mercury, which are cost-effective, rapid, and applicable to the environmental and biological milieus, is an important goal.10,11 Fluorescence techniques have become powerful tools for sensing and imaging Hg2+ ions because of their simplicity, high sensitivity and real-time monitoring with a short response time.12–15 To date, many excellent sensors have been developed for detection of Hg2+.16–21 Among them, dendritic light harvesting systems have attracted our attentions because of their unique structures, which can transfer the excitation energy of a multitude of peripheral chromophores to an energy acceptor core through an intramolecular FRET process.22–26 At the same time the fluorescent signals of the energy acceptor was amplified accompanying with the FRET process. This is a major requirement for the development of sensitive probes in the field of chemical sensors. Therefore, it is an emergency to develop a series of FRET-based sensors with high FRET efficiency.
Recently, dual-channel sensors have attracted more and more attentions due to their unique properties.27–29 Several dual-channel-based probes for Hg2+ and other ions have been reported.30,31 For example, dipyrromethene boron difluoride (BODIPY)-based dyes have obtained widespread concerns as dual-channel fluorescent probes,32,33 because of their remarkable properties, including high extinction coefficients, high quantum yields, intense absorption, low citotoxicity and good solubilities in many solvent systems.34–42 Perylene bisimide (PDI) have also generated great interest in the field of fluorescent sensors, owing to their excellent photochemical and chemical stability, high luminescence efficiency, high quantum yields, and good electron acceptors.43–45 Our research group has a longstanding interest in the synthesis and characterization of dendritic light-harvesting systems for some time.
In view of these, and as a part of our research interests in fluorescent sensors. Herein, we try to combine the advantages of PDI-based dendritic molecules with the BODIPY-based dyes to design a FRET-based ratiometric chemosensor. As a proof of concept, a novel dual-channel chemosensor to specifically sense Hg(II) ions by means of fluorescence and ultraviolet-visible absorption spectra has been designed and synthesized. In this probe, two different strategies for photoinduced electron transfer (PET) and fluorescence resonance energy transfer (FRET) have been designed and combined into one sensing system. As shown in Fig. 1-left, the PDI core serves as energy acceptor, the BODIPY-SS units serve as energy donor, and the reaction sites of PDI–BODIPY-SS with Hg2+ were assigned to the nitrogen atoms in triazole rings and the thiocarbonyls of BODIPY-SS,46,47 respectively. Therefore, the proposed sensing system represents dual recognition sites and segmented detection behaviors. The energy level diagram was used to illustrate the FRET processes of PDI–BODIPY-SS/CHO (Fig. 1-right). As one of the rare and suitable dual-channel probe for Hg2+, PDI–BODIPY-SS displayed a high sensitivity and remarkable selectivity to Hg2+ ions as compared with other metal ions. It affords a wide detection range for Hg2+ from 1 μM to 20 μM, a low detection limit of 12 nM, and a high FRET efficiency.
2. Results and discussion
2.1 The synthesis of dendritic probe PDI–BODIPY-SS
The syntheses of the dendritic probe PDI–BODIPY-SS, reference compounds BODIPY-SS and PDI-3 were showed in Schemes 1–3. The intermediate compound BODIPY-1 was prepared according to the standard synthetic procedures of BODIPY.48 Vilsmeier formylation using DMF–POCl3 gives compound BODIPY-CHO as the sole regio-isomer, as this product showed poor stability in air, it was used to the next reaction without further purification. Then, preparation of the formyl group using 1-propanethiol in the presence of ethanol leads to compound BODIPY-SS in 82% yield. The PDI core (PDI-3) with four terminal alkyne groups was synthesized according to a reported methods which was reported by our group for the first time.49 At last, compound PDI-3 reacted with intermediate BODIPY-SS to produce the target compound PDI–BODIPY-SS in the presence of sodium ascorbate and CuSO4·5H2O in a mixture of CHCl3, ethanol and water at room temperature. Compound PDI–BODIPY-SS not only have specific dual-channel fluorescent sensing for Hg2+ but also showed higher FRET efficiency with enhanced core’ emission for 5-folds as compared to that of the referenced molecule492.2 Hg(II)-induced FRET of probe PDI–BODIPY-SS
The recognition of the probe PDI–BODIPY-SS toward various metal ions such as Na+, K+, Cr3+, Mg2+, Co, Ni2+, Cu2+, Zn2+, Mg2+, Pd2+, Pb2+, Fe3+, Mn2+ and Al3+, were primarily investigated by UV-vis absorption spectra and fluorescence spectra in THF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
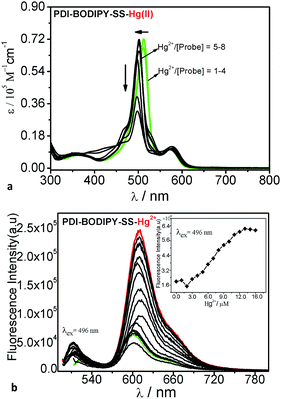
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) buffered with HEPES. None of these ions induced any significant changes. When 10 equivalents of Hg2+ were added to a water solution of probe PDI–BODIPY-SS (2 μM), there are important changes in the absorption spectra and fluorescence spectra. As shown in Fig. 2a and b, the probe PDI–BODIPY-SS showed two absorption bands at 510 nm and 576 nm, corresponding to the absorption bands of the BODIPY units and the PDI core in the compound PDI–BODIPY-SS. However, when 10 equivalents of Hg2+ was added to the solution of PDI–BODIPY-SS in THF/water, a new absorption peak at 496 nm appeared with 14 nm blue shift. Meanwhile, the corresponding fluorescence emission appeared a new peak at 510 nm and the color of solution changed from dark red to bright red as shown in the inset photos in Fig. 2.
9, v/v) buffered with HEPES. None of these ions induced any significant changes. When 10 equivalents of Hg2+ were added to a water solution of probe PDI–BODIPY-SS (2 μM), there are important changes in the absorption spectra and fluorescence spectra. As shown in Fig. 2a and b, the probe PDI–BODIPY-SS showed two absorption bands at 510 nm and 576 nm, corresponding to the absorption bands of the BODIPY units and the PDI core in the compound PDI–BODIPY-SS. However, when 10 equivalents of Hg2+ was added to the solution of PDI–BODIPY-SS in THF/water, a new absorption peak at 496 nm appeared with 14 nm blue shift. Meanwhile, the corresponding fluorescence emission appeared a new peak at 510 nm and the color of solution changed from dark red to bright red as shown in the inset photos in Fig. 2.
In addition, probe PDI–BODIPY-SS produced an enhanced emission peak centered at 610 nm. To assess the magnitude of the antenna effects in the system PDI–BODIPY-SS, we compared the emission intensities of PDI–BODIPY-SS (at 610 nm) when excited at the BODIPY antenna at 496 nm and directly excited at the PDI core at 576 nm. The antenna effects quantified in this way was 4 folds in the compound PDI–BODIPY-SS and 16 folds in the complex PDI–BODIPY-SS with Hg(II). These results indicate that the dendritic probe PDI–BODIPY-SS was a excellent light harvesting system with high FRET efficiency (99%).50,51 In order to study the selective behavior of the probe for Hg(II), the more detailed experiments and evidence will be showed below. The corresponding data was showed in Table 1.
| Compounds | λabsa (nm) | λemb (nm) | Δv1/Δv2c (cm−1) | Φ1/Φ2d | E1/E2e (%) |
|---|---|---|---|---|---|
| a Absorption maximum.b Emission maximum.c Stoke's shift = (1/λabs − 1/λem) × 107.d Fluorescence quantum yield determined in CHCl3 solution. Rhodamine 6G in ethanol (Φf = 0.95) was used as reference.e Energy transfer efficiency. | |||||
| PDI–BODIPY-SS | 510/576 | 530/610 | 1132/693 | 0.02/0.42 | 96 |
| PDI–BODIPY-CHO | 496/576 | 510/610 | 1132/693 | 0.02/0.72 | 99 |
| PDI–BODIPY-SS-H+ | 510/576 | 530/610 | 1132/693 | 0.02/0.52 | 96 |
| BODIPY-SS | 510 | 530 | 1132 | 0.84 | — |
2.3 Spectral-titration of probe PDI–BODIPY-SS with Hg(II)
The absorption spectra-titration of probe PDI–BODIPY-SS was showed in Fig. 3a. The free PDI–BODIPY-SS sensor exhibited absorption bands centered at 510 nm and 610 nm. When 1.0 to 4.0 equivalents of Hg2+ in water were added, the coordination effects of Hg2+ to probe PDI–BODIPY-SS resulted in a new absorption band centered at 496 nm. Meanwhile, absorption band centered at 510 nm disappeared gradually. Moreover, as the Hg2+ concentrations increased from 4.0 to 8.0 equivalents the intensity of the absorption peak at 496 nm gradually decreased. These phenomenons can be attributed to the dethioacetalization reaction between BODIPY-SS units and Hg2+ ions. The fluorescence titration of probe PDI–BODIPY-SS with Hg2+ ions were also tested. As shown in Fig. 3b, upon excitation at 496 nm, the free PDI–BODIPY-SS showed two weak emission peaks at 610 nm and 530 nm. The regular addition of Hg2+ ions resulted in the appearance of a strong emission peak at 610 nm and a new emission peak at 510 nm (Fig. 4). The addition of 4.0 equivalents of Hg2+ ions increased the emission intensity at 510 nm, and after 5–8 equivalents, the intensity of the peak at 530 nm disappeared (Fig. 3-left). At the same time the emission peak at 510 nm remained constant. Moreover, when the addition of 4–8 equivalents of Hg2+ ions, the emission intensity at 610 nm (excited at 496 nm) increased gradually and remained constant after the ratio of Hg2+/probe was more than 6.0 (Fig. 3 inset). This phenomenon was attributed to the coordination effect between Hg2+ ions and the nitrogen atoms in triazole rings, which disallowed electron transfer process from the electron donor to the PDI core. The changes of absorption and fluorescence prove that this probe have an excellent ability of dual-channel sensor for Hg2+ with segmented detection behaviors. | ||
| Fig. 4 Fluorescence intensities of probe PDI–BODIPY-SS at 510 nm as a function of Hg(II) concentration. | ||
The association constant (Ka) of PDI–BODIPY-SS with Hg2+ is 3.26 × 10−11 M, obtained by a nonlinear curve fitting of the fluorescence titration results.52 The inset in Fig. 3 exhibits the dependence of the intensity ratios of emission at 610 nm (Ff/Fi) on Hg2+ ions. This curve can be served as the calibration curve for the detection of Hg2+. The detection limit was also estimated from the titration results and was 1.2 × 10−8 M.53
For determination of stoichiometry between PDI–BODIPY-SS and Hg2+, job's plot analyses were used (Fig. 5). The method is that keeping total concentration of PDI–BODIPY-SS-SS and Hg2+ at 10 μM, and changing the molar ratio of Hg2+ from 0.1 to 0.9. When molar fraction of Hg2+ was 0.68, the emission intensity at 610 nm reached maximum, indicating that forming a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between Hg2+ and PDI–BODIPY-SS.
1 complex between Hg2+ and PDI–BODIPY-SS.
2.4 The possible dual-channel sensing mechanism of PDI–BODIPY-SS with Hg2+
As we discussed above, probe PDI–BODIPY-SS showed a dual-channel sensing process for Hg2+. With the addition of Hg2+ ions the dethioacetalization reaction between BODIPY-SS units and Hg2+ ions occurred in the first place (Scheme 4). When the ratio of Hg2+/probe ions was more than 4.0, the coordination reaction between Hg2+ and nitrogen atoms in triazole rings can be predicted according to the changes of spectral properties. In order to elucidate the mechanism, 1H NMR experiments were conducted. Fig. 6 showed the PDI–BODIPY-SS's 1H NMR changes in the absence and presence of Hg(II) ions. After addition of 4.0–6.0 equivalents of Hg(II), the protons beside the nitrogen atoms caused important downfield shifts. Especially for the protons in the triazole ring (0.26 ppm for proton b, 0.07 ppm for proton c, and 0.16 ppm for proton d) strongly suggested the participation of the nitrogen atoms in triazole rings in the coordination. But when the ratio exceed 6.0, no new changes of 1H NMR taken place. The new band at 9.82 ppm in the spectrum further more demonstrated the existence of C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) O.
O.
2.5 Selectivity of probe PDI–BODIPY-SS
High selectivity toward specific analyst over other competitive species is desired for any sensors. Therefore, potential interference from heavy transition metal ions were tested. As illustrated in Fig. 7a and b, upon the addition of various metal ions, probe PDI–BODIPY-SS displayed significantly enhanced fluorescence effects in the presence of Hg2+ and almost no obvious fluorescence changes from Na+, K+, Ca2+, Mg2+, Co, Ni2+, Cu2+, Zn2+, Mg2+, Pd2+, Pb2+, Fe3+, Mn2+ and Al3+ ions were observed. These results clearly showed that probe PDI–BODIPY-SS showed a good sensitivity and selectivity toward Hg2+ over other competitive metal ions. The selectivity of the prone towards the Hg(II) can be attributed to the coordination interaction of the bis-triazole and its nearby saturated nitrogen atoms with Hg(II). Meanwhile, the empty atomic orbitals of the Hg(II) happen to complementary with that of the bis-triazole in probe. However, the special electrons configuration of Hg(II) was different from that of other metal ions.2.6 FRET efficiency of probe PDI–BODIPY-SS at various pH values
In order to study the effects of the various pH values on the FRET efficiency of probe PDI–BODIPY-SS, the fluorescence changes of probe at various pH values were studied. As shown in Fig. 8, weak fluorescence was observed at pH > 7.0 (excited at 510 nm). However, as the pH values decreased from 7.0 to 2.0, emission band centered at 610 nm increased gradually and arrived a maximum at pH = 4.0, and no obvious changes can be obtained in the absorption spectra. Furthermore, the probe PDI–BODIPY-SS showed weaker fluorescent intensity (at 610 nm) and low FRET efficiency in acid solution compared with that of complex PDI–BODIPY-SS with Hg2+, which was due to the effects of different energy levels of the energy donors. The fluorescence responses of compound toward low pH indicate that it is a good probe for acidic organelles, such as lysosomes. Moreover, it can play as a selective subcellular localization.2.7 The possible bonding model
There are two important factors that impact the emission intensity of PDI core in PDI–BODIPY-SS. On the one hand, a PET process quenches the fluorescence emission of the PDI core.26,47 The bonding behaviors of Hg2+ ions with the nitrogen atoms in triazole rings would increase their oxidation potential, and disallowed the electron transfer. Consequently the emission would be “switched on”. The possible bonding behaviors of the FRET-based probe PDI–BODIPY-SS to Hg2+ ions were showed in Fig. 9a. On the other hand, the effects of energy levels of donors on the FRET efficiency of probe is also an important factor. Therefore, the energy levels of the energy donors–acceptor pairs were studied by DFT calculations at the B3LYP/6-31G(d) level of theory. The results (as shown in Fig. 9b) indicated that the energy level of BODIPY-CHO in the LUMO orbit (−2.13 eV) was more matched with that of PDI core in the LUMO orbits (−2.23 eV), compared with the LUMO level (−1.57 eV) of BODIPY-SS. These results of DFT calculations were consistent with the experimental results.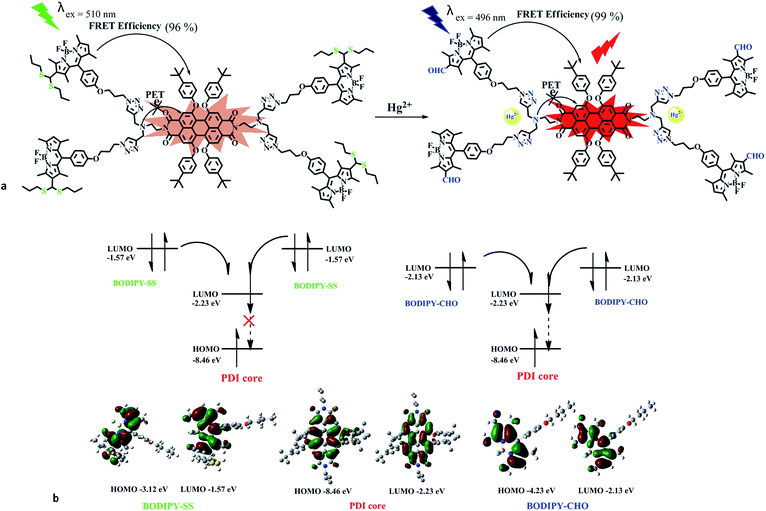 | ||
| Fig. 9 (a) The possible bonding model of probe PDI–BODIPY-SS with Hg(II). (b) The energy levels of energy donors–acceptor pairs in probe. | ||
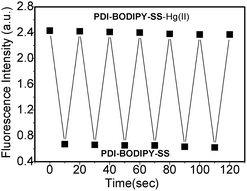
2.8 Reversibility of probe PDI–BODIPY-SS
The reversibility of the probe is a very important aspect when considering practical application. The interaction between PDI–BODIPY-SS and Hg2+ was reversible, which can be verified by fluorescence titration experiments of PDI–BODIPY-SS with NaOH solution. The results showed that the introduction of NaOH could lead to the decrease of fluorescence intensity of the system at 610 nm, when Hg2+ ions were added to the system again, the fluorescence of system recovered. Fig. 10 shows the fluorescence intensity changes with time upon switching from one solution to the other. This can be reversibly performed for at least six cycles.2.9 Fluorescence imaging via PDI–BODIPY-SS-staining
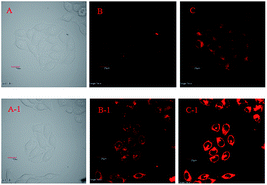
The intracellular Hg2+ imaging ability of PDI–BODIPY-SS was investigated on Hela cells with the laser confocal fluorescence microscope. The Hela cell lines were incubated with PDI–BODIPY-SS [1.0 μM in DMSO/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) buffered with HEPES, pH = 7.0] in a RPMI-1640 medium for 30 min at 37 °C and washed with a phosphate-buffered saline (PBS) buffer (pH = 7.2) to remove excess receptor PDI–BODIPY-SS. The cells display dim red fluorescence after stained by PDI–BODIPY-SS (Fig. 11A–C). Then the cells were treated with mercury perchlorate (8.0 μM) in the RPMI-1640 medium, incubated again for 15 min at 37 °C, and washed with a PBS buffer. The bright red fluorescence can be observed inside the cells (Fig. 11A-1–C-1), implying the Hg2+ concentration inside the cells has been enhanced. These results suggest that probe PDI–BODIPY-SS is an effective FRET-based intracellular Hg(II) imaging agent.
9, v/v) buffered with HEPES, pH = 7.0] in a RPMI-1640 medium for 30 min at 37 °C and washed with a phosphate-buffered saline (PBS) buffer (pH = 7.2) to remove excess receptor PDI–BODIPY-SS. The cells display dim red fluorescence after stained by PDI–BODIPY-SS (Fig. 11A–C). Then the cells were treated with mercury perchlorate (8.0 μM) in the RPMI-1640 medium, incubated again for 15 min at 37 °C, and washed with a PBS buffer. The bright red fluorescence can be observed inside the cells (Fig. 11A-1–C-1), implying the Hg2+ concentration inside the cells has been enhanced. These results suggest that probe PDI–BODIPY-SS is an effective FRET-based intracellular Hg(II) imaging agent.
3. Conclusions
In conclusion, a novel dual-channel dendritic chemosensor PDI–BODIPY-SS was designed and synthesized. Specific dual-channel spectral responses of probe PDI–BODIPY-SS toward Hg2+ were investigated in aqueous solution, which can be deduced to the dethioacetalization reaction of BODIPY-SS promoted selectively by Hg2+ ions and the coordination reaction between Hg2+ and the nitrogen atoms in triazole rings of PDI–BODIPY-SS. The possible mechanism has been confirmed by 1H NMR. The probe PDI–BODIPY-SS can selectively recognize Hg2+ over other metal ions including Na+, K+, Ca2+, Mg2+, Co2+, Ni2+, Cu2+, Zn2+, Mg2+, Pd2+, Pb2+, Mn2+ and Al3+ in aqueous solution. The favorable features of PDI–BODIPY-SS toward Hg2+ included high selectivity, low detection limit (12 nM), rapid response time (4–8 s), and significant fluorescence enhancement with high intramolecular FRET efficiency (99%) between the BODIPY-CHO units and PDI core. In addition, in order to study the effects of energy levels of donors on the FRET efficiency of probe PDI–BODIPY-SS, the DFT calculations at the B3LYP/6-31G(d) level of theory were adopted, The results indicated that the energy levels of BODIPY-CHO in the LUMO orbit were more matched with that of PDI core in the LUMO orbits when compared with that of BODIPY-SS. Confocal fluorescence imaging of cells for detecting Hg2+ in vivo was carried out successfully.4. Experimental
4.1 Materials and methods
1H NMR and 13C NMR spectra were recorded on a Bruker DMX 300 NMR spectrometer in CDCl3 with tetramethylsilane (TMS) as internal standard. Chemical shifts are given in parts per million (ppm). High resolution mass spectrum (HRMS) were recorded on an Ultraflex II MALDI-TOF mass spectrometer, which was used only for the compound PDI–BODIPY-SS duo to its high cost. Mass spectra of other compounds were recorded on Agilent 1100 LC-MSD. UV-visible absorption spectra were determined on a Shimadu UV-3600 spectrophotometer. Fluorescence spectra were measured on a HORIBA FL-4 Max spectrometer. FT-IR spectra were recorded on a Nicolet 750 series in the region of 4000–400 cm−1 using KBr pellets. All reagents used were purchased from Aldrich, Fluka or Alfa Aesar. Anhydrous dichloromethane were used in the synthesis of compound BODIPY-1. Solvents used in spectroscopic measurements were analytical grade. Reactions were monitored by thin layer chromatography using Merck TLC Silica gel 60 F254. Silica gel column chromatography was performed over Merck Silica gel 60. DFT calculations of compounds were performed using the Gaussian 03 program package and were optimized at the B3LYP/6-31G(d) level of theory. The molecular orbitals of compounds were visualized using Gauss view.4.2 Synthesis
The synthetic routes adopted for preparation of PDI–BODIPY-SS were shown in Schemes 1–3. The detailed synthesis methods of compound QN-NH2 and compound CHO-N3 were in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give a red solid 1.42 g, yield: 82% (mp 248–250 °C). FT-IR (KBr) cm−1: 3422 (νNH); 2950–2863 (νCH); 1698 (νasN–C
1) to give a red solid 1.42 g, yield: 82% (mp 248–250 °C). FT-IR (KBr) cm−1: 3422 (νNH); 2950–2863 (νCH); 1698 (νasN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1664 (νsN–C
O); 1664 (νsN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1576 (νN–C
O); 1576 (νN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (CDCl3, ppm): δ 8.67 (s, 4H), 4.38–4.34 (t, 4H, J = 6 Hz), 3.56 (s, 8H), 3.01–2.97 (t, 4H, J = 6 Hz), 2.18 (s, 4H). 13C NMR (CDCl3, ppm): δ 162.3, 135.3, 132.9, 132.9, 131.4, 128.6, 123.3, 123.2, 73.0, 50.2, 42.5, 38.3. TOF-MS-ES: m/z. Calculated: [M + H]+ = 765.1, found: 765.1.
O). 1H NMR (CDCl3, ppm): δ 8.67 (s, 4H), 4.38–4.34 (t, 4H, J = 6 Hz), 3.56 (s, 8H), 3.01–2.97 (t, 4H, J = 6 Hz), 2.18 (s, 4H). 13C NMR (CDCl3, ppm): δ 162.3, 135.3, 132.9, 132.9, 131.4, 128.6, 123.3, 123.2, 73.0, 50.2, 42.5, 38.3. TOF-MS-ES: m/z. Calculated: [M + H]+ = 765.1, found: 765.1.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) 1660 (νsN–C
O) 1660 (νsN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) 1587 (νN–C
O) 1587 (νN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (CDCl3, ppm): δ 8.23 (s, 4H, b), 7.25–7.22 (d, 8H, J = 9 Hz, a′), 6.84–6.81 (d, 8H, J = 9 Hz, a), 4.30–4.25 (t, 4H, J = 9 Hz, e), 3.54 (s, 8H, g), 2.93–2.89 (t, 4H, J = 6 Hz, f), 2.17 (s, 4H), 1.30 (s, 36H, –C(CH3)3). 13C NMR (CDCl3, ppm): δ 146.3, 142.8, 139.4, 138.3, 132.8, 128.5, 127.6, 124.8, 123.5, 116.2, 109.2, 65.0, 64.9, 34.7, 32.0.
O). 1H NMR (CDCl3, ppm): δ 8.23 (s, 4H, b), 7.25–7.22 (d, 8H, J = 9 Hz, a′), 6.84–6.81 (d, 8H, J = 9 Hz, a), 4.30–4.25 (t, 4H, J = 9 Hz, e), 3.54 (s, 8H, g), 2.93–2.89 (t, 4H, J = 6 Hz, f), 2.17 (s, 4H), 1.30 (s, 36H, –C(CH3)3). 13C NMR (CDCl3, ppm): δ 146.3, 142.8, 139.4, 138.3, 132.8, 128.5, 127.6, 124.8, 123.5, 116.2, 109.2, 65.0, 64.9, 34.7, 32.0.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH2–), 3.54 (t, 2H, J = 6.00 Hz, –C
2–CH2–), 3.54 (t, 2H, J = 6.00 Hz, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–N3), 2.55 (s, 6H), 2.12–2.07 (m, 2H, CH2C
2–N3), 2.55 (s, 6H), 2.12–2.07 (m, 2H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2), 1.43 (s, 6H). 13C NMR (CDCl3, ppm): δ 190.5, 163.5, 131.7, 114.6, 68.8, 65.5, 34.7, 31.9, 29.5. TOF-MS-ES: m/z. Calculated: ([M + Na])+ = 446.2, found: 446.2, ([M])+ = 424.2, found: 424.2.
2CH2), 1.43 (s, 6H). 13C NMR (CDCl3, ppm): δ 190.5, 163.5, 131.7, 114.6, 68.8, 65.5, 34.7, 31.9, 29.5. TOF-MS-ES: m/z. Calculated: ([M + Na])+ = 446.2, found: 446.2, ([M])+ = 424.2, found: 424.2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). A yellow solid got 0.48 g, yield: 80%. 1H NMR (CDCl3, ppm): δ 7.17 (d, 2H, J = 8.40 Hz), 7.04 (d, 2H, J = 8.40 Hz), 5.98 (s, 1H), 4.86 (s, 1H), 4.13–4.09 (m, 2H), 3.58–3.53 (m, 2H), 2.51–2.48 (m, 3H), 1.65–1.60 (m, 5H), 2.70 (s, 3H), 2.55 (s, 3H), 2.12–2.08 (m, 2H), 1.48 (s, 3H), 1.41 (s, 3H), 1.01–0.92 (m, 6H, –CH2CH2C
1). A yellow solid got 0.48 g, yield: 80%. 1H NMR (CDCl3, ppm): δ 7.17 (d, 2H, J = 8.40 Hz), 7.04 (d, 2H, J = 8.40 Hz), 5.98 (s, 1H), 4.86 (s, 1H), 4.13–4.09 (m, 2H), 3.58–3.53 (m, 2H), 2.51–2.48 (m, 3H), 1.65–1.60 (m, 5H), 2.70 (s, 3H), 2.55 (s, 3H), 2.12–2.08 (m, 2H), 1.48 (s, 3H), 1.41 (s, 3H), 1.01–0.92 (m, 6H, –CH2CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3). 13C NMR (CDCl3, ppm): δ 159.3, 129.3, 129.0, 127.3, 115.3, 64.7, 48.2, 43.5, 35.1, 28.8, 22.7, 14.7, 12.6. TOF-MS-ES: m/z. Calculated: ([M + H])+ = 586.3, found: 586.3.
3). 13C NMR (CDCl3, ppm): δ 159.3, 129.3, 129.0, 127.3, 115.3, 64.7, 48.2, 43.5, 35.1, 28.8, 22.7, 14.7, 12.6. TOF-MS-ES: m/z. Calculated: ([M + H])+ = 586.3, found: 586.3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was stirred at room temperature for 48 h. The solvents were removed under reduced pressure and the crude product was purified by column chromatography (CHCl3/methanol = 9
1) was stirred at room temperature for 48 h. The solvents were removed under reduced pressure and the crude product was purified by column chromatography (CHCl3/methanol = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), a red solid got 0.81 g, yield: 82% (mp 164–168 °C). FT-IR (KBr) cm−1: 3422 (νNH); 2964–2870 (νCH); 1698 (νasN–C
1), a red solid got 0.81 g, yield: 82% (mp 164–168 °C). FT-IR (KBr) cm−1: 3422 (νNH); 2964–2870 (νCH); 1698 (νasN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1657 (νsN–C
O); 1657 (νsN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1550 (νN–C
O); 1550 (νN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (CDCl3, ppm): δ 8.23 (s, 4H), 7.68 (s, 4H), 7.24 (d, 8H, J = 8.10 Hz), 7.14 (d, 8H, J = 8.10 Hz), 6.96 (d, 8H, J = 8.10 Hz), 6.84 (d, 8H, J = 8.10 Hz), 5.97 (s, 4H), 4.86 (s, 4H), 4.52–4.56 (m, 8H), 4.33 (s, 4H), 3.99–4.02 (m, 8H), 3.85 (s, 8H), 2.70 (s, 12H), 2.55 (s, 24H), 2.51–2.50 (m, 12H), 1.60–1.53 (m, 20H), 1.47 (s, 12H), 1.39 (s, 12H), 1.29 (s, 36H, –C(C
O). 1H NMR (CDCl3, ppm): δ 8.23 (s, 4H), 7.68 (s, 4H), 7.24 (d, 8H, J = 8.10 Hz), 7.14 (d, 8H, J = 8.10 Hz), 6.96 (d, 8H, J = 8.10 Hz), 6.84 (d, 8H, J = 8.10 Hz), 5.97 (s, 4H), 4.86 (s, 4H), 4.52–4.56 (m, 8H), 4.33 (s, 4H), 3.99–4.02 (m, 8H), 3.85 (s, 8H), 2.70 (s, 12H), 2.55 (s, 24H), 2.51–2.50 (m, 12H), 1.60–1.53 (m, 20H), 1.47 (s, 12H), 1.39 (s, 12H), 1.29 (s, 36H, –C(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)3), 0.97–0.92 (m, 24H). 13C NMR (CDCl3, ppm): δ 163.3, 159.0, 155.9, 152.9, 147.4, 144.2, 141.5, 132.9, 131.7, 129.3, 127.5, 126.7, 123.6, 122.4, 121.1, 120.6, 119.9, 119.3, 115.1, 64.3, 47.7, 46.9, 34.4, 31.4, 29.9, 14.6. MALDI-TOF-MS: calculated: m/z [M + H]+ = 3562.6051, found: 3562.6051.
3)3), 0.97–0.92 (m, 24H). 13C NMR (CDCl3, ppm): δ 163.3, 159.0, 155.9, 152.9, 147.4, 144.2, 141.5, 132.9, 131.7, 129.3, 127.5, 126.7, 123.6, 122.4, 121.1, 120.6, 119.9, 119.3, 115.1, 64.3, 47.7, 46.9, 34.4, 31.4, 29.9, 14.6. MALDI-TOF-MS: calculated: m/z [M + H]+ = 3562.6051, found: 3562.6051.Acknowledgements
We sincerely acknowledge Miss H. L. Liu and Mr Y. Yang for the confocal fluorescence images. This work was financially supported by the Fundamental Research Funds for the National Natural Science Foundation of China (No. 61178057) and the Central Universities (No. CXLX12_0085).Notes and references
- N. Kumar, V. Bhalla and M. Kumar, Analyst, 2014, 139, 543–558 RSC.
- X. Y. Guan, W. Y. Lin and W. M. Huang, Org. Biomol. Chem., 2014, 12, 3944–3949 CAS.
- Y. H. Lee, M. H. Lee, J. F. Zhang and J. S. Kim, J. Org. Chem., 2010, 75, 7159–7165 CrossRef CAS PubMed.
- G. W. Chen, F. L. Song, X. Q. Xiong and X. J. Peng, Ind. Eng. Chem. Res., 2013, 52, 11228–11245 CrossRef CAS.
- Z. Kostereli, T. Ozdemir, O. Buyukcakir and E. U. Akkaya, Org. Lett., 2012, 14, 3636 CrossRef CAS PubMed.
- M. Deniz, O. Yilmaz, A. Bozdemir and E. U. Akkaya, Org. Lett., 2006, 8, 2871–2876 CrossRef PubMed.
- G. H. Zheng, Y. M. Guo and W. H. Li, J. Am. Chem. Soc., 2007, 129, 10616–10617 CrossRef CAS PubMed.
- Z. G. Gerardo, F. Michael, R. Regis, S. Nathalie, D. Jean and R. Ernesto, J. Phys. Chem. C, 2014, 118, 8280–8294 Search PubMed.
- R. Bergonzi, L. Fabbrizzi, M. Licchelli and C. Mangano, Coord. Chem. Rev., 1998, 170, 31–46 CrossRef CAS.
- R. D. Hancock, D. L. Melton, J. M. Harrington, F. C. McDonald, R. T. Gephart, L. L. Boone, S. B. Jones, N. E. Dean, J. R. Whitehead and G. M. Cockrell, Coord. Chem. Rev., 2007, 251, 1678–1689 CrossRef CAS PubMed.
- J. F. Zhang, Y. Zhou, J. Yoon and J. S. Kim, Chem. Soc. Rev., 2011, 40, 3416–3429 RSC.
- Z. Xu, J. Yoon and D. R. Spring, Chem. Commun., 2010, 46, 2563–2565 RSC.
- D. K. Zhang, V. MartÍn, I. GarcÍa-Moreno, A. Costela, M. E. Pėrez-Ojeda and Y. Xiao, Phys. Chem. Chem. Phys., 2011, 13, 13026–13033 RSC.
- X. F. Guo, X. H. Qian and L. H. Jia, J. Am. Chem. Soc., 2004, 126, 2272–2273 CrossRef CAS PubMed.
- M. J. Culzoni, A. Muñoz de la Peña, A. Machuca, H. C. Goicoechea and R. Babiano, Anal. Methods, 2013, 5, 30–49 RSC.
- K. L. Liu, Z. J. Xu, M. Z. Yin, W. T. Yang, B. C. He, W. Wei and J. Shen, J. Mater. Chem. B, 2014, 2, 2093–2096 RSC.
- H. Son, J. H. Lee, Y. R. Kim, I. S. Lee, S. Y. Han, X. G. Liu, J. Jaworski and J. H. Jung, Analyst, 2012, 137, 3914–3916 RSC.
- T. K. Khan and M. Ravikanth, Dyes Pigm., 2012, 95, 89–95 CrossRef CAS PubMed.
- X. X. He, J. Zhang, X. G. Liu, L. Dong, D. Li, H. Y. Qiu and S. C. Yin, Sens. Actuators, B, 2014, 192, 29–35 CrossRef CAS PubMed.
- X. J. Jiang, C. L. Wong, P. C. Lo and D. K. P. Ng, Dalton Trans., 2012, 41, 1801–1807 RSC.
- D. Y. Kim, K. Yamamoto and K. H. Ahn, Tetrahedron, 2012, 68, 5279–5282 CrossRef CAS PubMed.
- Z. G. Gerardo, F. Michael, R. Regis, S. Nathalie, D. Jean and R. Ernesto, J. Phys. Chem. C, 2014, 118, 8280–8294 Search PubMed.
- R. Ponnapati, M. J. Felipe and R. Advincula, Macromolecules, 2011, 44, 7530–7537 CrossRef CAS.
- P. Singh, L. S. Mittal, V. Vanita, R. Kumar and G. Bhargava, Chem. Commun., 2014, 50, 13994–13997 RSC.
- K. R. Wang, H. W. An, Y. Q. Wang, C. G. Zhang and X. L. Li, Org. Biomol. Chem., 2013, 11, 1007–1012 CAS.
- H. R. Cheng and Y. Qian, Dyes Pigm., 2015, 112, 317–326 CrossRef CAS PubMed.
- S. R. Patil, J. P. Nandre, D. Jadhav, S. Bothra, S. K. Sahoo, M. Devi, C. P. Pradeep, P. P. Mahulikar and U. D. Patil, Dalton Trans., 2014, 43, 13299–13306 RSC.
- A. F. Liu, L. Yang, Z. Y. Zhang, Z. L. Zhang and D. M. Xu, Dyes Pigm., 2013, 99(2), 472–479 CrossRef CAS PubMed.
- Y. M. Zhang, W. J. Qu, G. Y. Gao, B. B. Shi, G. Y. Wu, T. B. Wei, Q. Lin and H. Yao, New J. Chem., 2014, 38, 5075–5080 RSC.
- X. X. He, J. Zhang, X. G. Liu, L. Dong, D. Li, H. Y. Qiu and S. C. Yin, Sens. Actuators, B, 2014, 192(1), 29–35 CrossRef CAS PubMed.
- X. J. Xie and Y. Qin, Sens. Actuators, B, 2011, 156, 213–217 CrossRef CAS PubMed.
- X. G. He, J. Zhang, X. G. Liu, L. Dong, D. Li, H. Y. Qiu and S. C. Yin, Sens. Actuators, B, 2014, 192, 29–35 CrossRef CAS PubMed.
- X. J. Zhang, Y. F. Xu, P. Guo and X. H. Qian, New J. Chem., 2012, 36, 1621–1625 RSC.
- D. D. Su, C. L. Teoh, S. Sahu and R. K. Das, Biomaterials, 2014, 35, 6078–6085 CrossRef CAS PubMed.
- X. T. Jing, F. B. Yu and L. X. Chen, Chem. Commun., 2014, 50, 14253–14256 RSC.
- G. G. Dias, B. L. Rodrigues, J. M. Resende, H. D. R. Calado and C. A. de Simone, et al., Chem. Commun., 2015, 51, 9141–9144 RSC.
- R. S. Singh, R. K. Gupta, R. P. Paitandi, A. Misra and D. S. Pandey, New J. Chem., 2015, 39, 2233–2239 RSC.
- P. Majumdar, J. Mack and T. Nyokong, RSC Adv., 2015, 5, 78253–78258 RSC.
- B. Umasekhar, E. Ganapathi, T. Chatterjee and M. Ravikanth, Dalton Trans., 2015, 44, 16516–16527 RSC.
- J. W. Tian, J. F. Zhou, Z. Shen, L. Ding, J. S. Yu and H. X. Ju, Chem. Sci., 2015, 6, 5969–5977 RSC.
- M. Vedamalai and S. P. Wu, Org. Biomol. Chem., 2012, 10, 5410–5416 CAS.
- L. Y. Wang, G. Fang and D. Cao, Sens. Actuators, B, 2015, 207, 849–857 CrossRef CAS PubMed.
- J. A. A. Elemans, R. van Hameren, R. J. M. Nolte and A. E. Rowan, Adv. Mater., 2006, 18, 1251–1266 CrossRef CAS PubMed.
- M. R. Wasielewski, J. Org. Chem., 2006, 71, 5051–5066 CrossRef CAS PubMed.
- F. WÜrthner, Chem. Commun., 2004, 1564–1579 RSC.
- L. L. Song, Z. H. Lei, B. Y. Zhang, Z. P. Xu, Z. Li and Y. J. Yang, Anal. Methods, 2014, 6, 7597–7600 RSC.
- S. Saha, H. Agarwalla, H. Gupta, M. Baidya, E. Suresh, S. K. Ghosh and A. Das, Dalton Trans., 2013, 42, 15097–15105 RSC.
- M. Baruah, W. W. Qin, R. A. Vallèe, T. Rohand, W. Dehaen and N. Boens, Org. Lett., 2005, 7, 4377–4380 CrossRef CAS PubMed.
- H. R. Cheng and Y. Qian, Sens. Actuators, B, 2015, 219, 57–64 CrossRef CAS PubMed.
- N. I. Georgiev, A. M. Asiri, A. H. Qusti, K. A. Alamry and V. B. Bojinov, Dyes Pigm., 2014, 102, 35–45 CrossRef CAS PubMed.
- N. I. Georgiev, A. M. Asiri, A. H. Qusti, K. A. Alamry and V. B. Bojinov, Sens. Actuators, B, 2014, 190, 185–198 CrossRef CAS PubMed.
- N. I. Georgiev, A. R. Sakr and V. B. Bojinov, Dyes Pigm., 2011, 91, 332–339 CrossRef CAS PubMed.
- B. C. Zhu, C. C. Gao, Y. Z. Zhao, C. Y. Liu, Y. Li, Q. Wei, Z. M. Ma, B. Du and X. L. Zhang, Chem. Commun., 2011, 47, 8656–8658 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17664f |
| This journal is © The Royal Society of Chemistry 2015 |