Glycerol acetins: fuel additive synthesis by acetylation and esterification of glycerol using cesium phosphotungstate catalyst†
Abstract
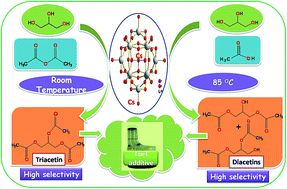
Glycerol acetylation and esterification reactions with acetic anhydride and acetic acid respectively give acetins, in which di and tri acetins are commercially important products used as fuel additives. Acetylation and esterification of glycerol were studied over various solid acid catalysts namely, cesium phosphotungstate, amberlyst-15, H-beta, sulfated zirconia and montmorillonite K-10 under mild reaction conditions. The catalysts were characterized by XRD, FTIR, SEM and acidity measurements. Among all the catalysts evaluated in this study, cesium phosphotungstate showed highest activity with >98% conversion for both the reactions, whereas di and triacetins selectivity was 99.1% for acetylation and 75% for esterification reaction. The catalyst with high Brönsted acidity gave high activity for both the reactions, whereas selectivity for di and tri acetins depends on nature of active sites.

 Please wait while we load your content...
Please wait while we load your content...