Antioxidant and hepatoprotective effects of a pigment–protein complex from Chlorella vulgaris on carbon tetrachloride-induced liver damage in vivo
Xixi Caiab,
Qian Yangb and
Shaoyun Wang*b
aCollege of Chemistry, Fuzhou University, Fuzhou 350108, China
bCollege of Biological Science and Technology, Fuzhou University, Fuzhou 350108, China. E-mail: shywang@fzu.edu.cn; Fax: +86-591-22866278; Tel: +86-591-22866375
First published on 22nd October 2015
Abstract
The antioxidant and hepatoprotective activities of pigments in the natural form of a pigment–protein complex were investigated. Pigment–protein complex (PPC) was isolated from Chlorella vulgaris through thylakoid protein solubilization, anion exchange chromatography and gel filtration chromatography. PPC possessed a chlorophyll![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lutein
lutein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) protein ratio of 94
protein ratio of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 153
153![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (w/w/w) according to HPLC and spectrophotometric analysis. Various antioxidant evaluation systems were used to evaluate the antioxidant activity of PPC in vitro. Results showed that PPC exhibited significant DPPH radical scavenging activity with an IC50 of 313 μg mL−1. Distinct Fe2+ ion chelating activity, reducing capacity and lipid peroxidation inhibition activity were observed at a concentration of 1 mg mL−1. For the hepatoprotective effects, administration of PPC at different concentrations (50 and 100 mg kg−1 BW) could significantly decrease the carbon tetrachloride-induced elevation of the hepatosomatic index. Increased serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were attenuated with PPC pretreatment. In addition, PPC effectively restored suppressed hepatic superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) activities. Moreover, PPC significantly reduced the formation of malondialdehyde (MDA). The results obtained from this study clearly verified the hepatoprotective effect of PPC in CCl4-induced hepatotoxicity in vivo, suggesting that PPC has the potential to be exploited as a dietary supplement against free radical oxidation which could enhance resistivity against oxidative stress in the human body.
100 (w/w/w) according to HPLC and spectrophotometric analysis. Various antioxidant evaluation systems were used to evaluate the antioxidant activity of PPC in vitro. Results showed that PPC exhibited significant DPPH radical scavenging activity with an IC50 of 313 μg mL−1. Distinct Fe2+ ion chelating activity, reducing capacity and lipid peroxidation inhibition activity were observed at a concentration of 1 mg mL−1. For the hepatoprotective effects, administration of PPC at different concentrations (50 and 100 mg kg−1 BW) could significantly decrease the carbon tetrachloride-induced elevation of the hepatosomatic index. Increased serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were attenuated with PPC pretreatment. In addition, PPC effectively restored suppressed hepatic superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) activities. Moreover, PPC significantly reduced the formation of malondialdehyde (MDA). The results obtained from this study clearly verified the hepatoprotective effect of PPC in CCl4-induced hepatotoxicity in vivo, suggesting that PPC has the potential to be exploited as a dietary supplement against free radical oxidation which could enhance resistivity against oxidative stress in the human body.
1. Introduction
Free radicals such as the superoxide anion radical (˙O2−) and hydroxyl radical (˙OH) are highly reactive species with single and unbalanced electrons, which are involved in the oxidation process of biological molecules, and will cause many adverse effects in food and biological systems.1 In human organs, free radicals, which are inevitably produced through oxidative metabolism, induce several diseases like arteriosclerosis and cancer. Liver damage is a widespread disease which can be caused by several compounds, such as ethanol, CCl4 and bromobenzene. The mechanism of liver injury induced by chemical compounds is thought to involve excess free radical generation and lipid peroxidation.2 It has been a hot topic in the field of biochemical nutrition to search for safe antioxidants to prevent free radical-induced damage.3Natural pigments, extracted from animals, plants and microorganisms, are one of the most important antioxidant systems that can neutralize free radicals in the cells.4,5 It was shown that high radiant energy increased carotenoid concentration in cells, presumably to prevent photooxidation6 and some carotenoids strongly contributed to the protection of plants against photooxidative damage as direct quenchers of reactive oxygen species (ROS).7 The antioxidant activity of carotenoids such as luteins may be due to the conjugated double bonds and the phenolic hydroxyl groups at both ends of their chemical structures.8 Chlorophylls are also important antioxidants. They can act as lipid antioxidants in stored edible oils9 and prevent oxidative DNA damage and lipid peroxidation both by reducing ROS and chelating metal ions.10 However, solvent extraction is the main industrially applicable method for pigment extraction,11 and consequently, the massive use of organic solvents is not environmentally friendly and destroys the natural matrix of the pigments. Actually, hydrophobic pigments in cells are found associated with the hydrophobic domains of lipid–protein complexes,12 and they play a role in photosynthesis in the form of light-harvesting complexes (LHCs) in most plants and algae.13 Previous studies of LHCs mainly focused on their pigment composition, structure and energy transfer,14,15 and by contrast, the functional activities of pigments in the natural form of the pigment–protein complex are rarely reported.
Marine algae, which have traditionally formed part of the Oriental diet, especially in China, Japan and Korea, are becoming a hot research topic because of their biological components.16 Chlorella vulgaris, which belongs to the marine microalgae, contains a large number of active substances which are beneficial to the human body, such as pigment, fat, unsaturated fatty acids, proteins, polysaccharides, squalene etc. It is usually composed of 0.2–0.5% lutein, 2–4% chlorophyll and the protein content reaches up to 50% (dry weight).17 It would be interesting to isolate the natural matrix of pigments through aqueous extraction instead of organic solvent extraction, and investigate the activities of pigments in the natural form of the pigment–protein complex (PPC). Therefore, this study aims to isolate the natural pigment–protein complex from Chlorella vulgaris and evaluate its antioxidant activities in vitro and hepatoprotective effects in CCl4-induced hepatotoxicity in vivo. The study is of significance in suggesting that the natural protein-based pigment complex has potential for making dietary supplements against oxidation and in increasing resistivity against oxidative stress in the human body.
2. Materials and methods
2.1. Materials
The algae Chlorella vulgaris STIO02 was kindly provided by Third Institute of Oceanography, State Oceanic Administration, P. R. China and was stored at −20 °C before use.Toyopearl DEAE-650M was the product of TOSOH Co. (Japan). Sephadex G-50 Fine was purchased from GE Healthcare (USA). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS) and lutein were obtained from Sigma Chemical Co. (USA). The Bicinchoninic acid (BCA) protein kit and all the kits for the biochemical indexes analysis used in the animal experiment were the products of Nanjing Jiancheng Bioengineering Institute (China). Methanol and acetonitrile used in the liquid chromatography were of HPLC grade. All other chemicals and reagents used were of analytical grade and commercially available.
2.2. Solubilization of thylakoid proteins
The Chlorella vulgaris algae were lysed in phosphate buffer (20 mM, pH 7.0) by ultrasonication in an ice bath. Cell debris was removed by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C. Then a 25% saturated ammonium sulfate precipitation was applied to collect the membranes. Here, CHAPS, a zwitterionic detergent that can effectively maintain protein activity, was used to solubilize the intrinsic membrane-associated pigment–protein complex. The membrane fraction was re-suspended in Tris–HCl buffer (20 mM, pH 8.0) and 1% CHAPS was added. The mixture was stirred in the dark for 1 h at 4 °C and then centrifuged at 12
000 rpm for 10 min at 4 °C. Then a 25% saturated ammonium sulfate precipitation was applied to collect the membranes. Here, CHAPS, a zwitterionic detergent that can effectively maintain protein activity, was used to solubilize the intrinsic membrane-associated pigment–protein complex. The membrane fraction was re-suspended in Tris–HCl buffer (20 mM, pH 8.0) and 1% CHAPS was added. The mixture was stirred in the dark for 1 h at 4 °C and then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 30 min. The supernatant was collected for further purification.
500 rpm for 30 min. The supernatant was collected for further purification.
2.3. Isolation and purification of pigment–protein complex
A Toyopearl DEAE-650M column (Φ 1.6 × 20 cm) was previously equilibrated with Tris–HCl buffer (20 mM, pH 8.0) containing 8 mM of CHAPS. The solubilized thylakoid proteins were loaded onto the column and then washed with the same buffer followed by a linear gradient of 0–0.5 M NaCl with a flow rate of 0.5 mL min−1 and 10 min tube−1. The absorbances of all fractions were monitored at 430 nm and 280 nm. The pigment protein fractions were pooled and concentrated by ultrafiltration. The concentrate was then applied to a Sephadex G-50 column (Φ 1.6 × 100 cm) that was pre-equilibrated with Tris–HCl buffer (20 mM, pH 8.0) containing 8 mM of CHAPS and 50 mM of NaCl. The column was used with the same buffer and the flow rate was 0.3 mL min−1. The absorbance of the fractions was monitored and the eluted fractions containing the pigment–protein complex were collected.2.4. Characterization of pigment–protein complex
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min was applied. The absorbance of the supernatant at 645 and 663 nm was measured. Chlorophyll content was calculated according to the following equation:
000 rpm for 5 min was applied. The absorbance of the supernatant at 645 and 663 nm was measured. Chlorophyll content was calculated according to the following equation:| Chlorophyll (μg mL−1) = 20.2A645 + 8.02A663. |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to removed the pigments. The pellet was re-suspended in 50 μL of 2% (w/v) SDS and then heated at 60 °C for 30 min. The protein content was quantified using a bicinchoninic acid kit according to the protocol.
000 rpm for 5 min to removed the pigments. The pellet was re-suspended in 50 μL of 2% (w/v) SDS and then heated at 60 °C for 30 min. The protein content was quantified using a bicinchoninic acid kit according to the protocol.2.5. Antioxidant activity in vitro
| DPPH radical scavenging activity (%) = (Acontrol − Asample)/Acontrol × 100, |
| Metal chelating activity (%) = (Acontrol − Asample)/Acontrol × 100, | (1) |
2.6. Effect on CCl4-induced hepatic injury in mice
2.7. Statistical analysis
All data are presented as means ± standard deviation (SD). Statistical evaluation was carried out with IBM SPSS 19.0 software. Comparisons of multiple treatment conditions were analyzed using one-way analysis of variance (ANOVA) with Duncan’s test for post hoc analysis and p < 0.05 values were considered as statistically significant.3. Results and discussion
3.1. Isolation of pigment–protein complex from Chlorella
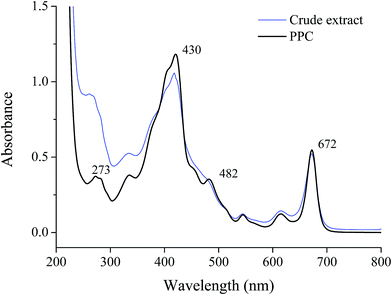
A crude pigment–protein complex extract of Chlorella was obtained by aqueous extraction with the addition of CHAPS and chromatography was employed to improve the purity of the pigment–protein complex. Through DEAE anion-exchange chromatography, the crude extract was divided into four fractions (Fig. 1a). Fraction P2 exhibited an obvious absorbance at both 280 nm and 430 nm and was dark green. For further purification, fraction P2 was concentrated by ultrafiltration and applied to a Sephadex G-50 column. Three fractions were obtained as shown in Fig. 1b. The pigment–protein complex was mainly present in fraction P2-2 while the other fractions contained only proteins. The absorbance spectrum of P2-2 is shown in Fig. 2. The profile was slightly similar to that of the crude extract, indicating the reasonable recovery achieved after isolation through chromatography. The profile included obvious chlorophyll absorbance at 430 and 672 nm. The peak at 482 nm indicated the presence of lutein when compared with the standard marker pigments (data not shown).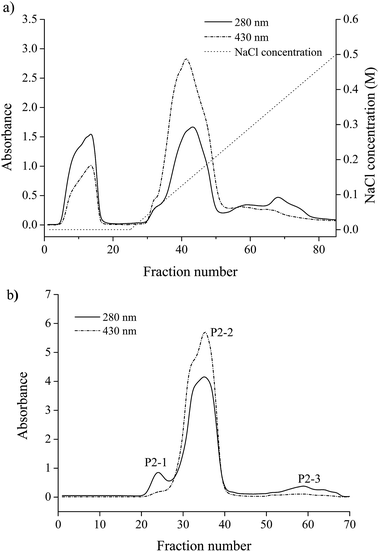 | ||
| Fig. 1 Purification of the pigment–protein complex from Chlorella. (a) DEAE anion-exchange chromatography, (b) Sephadex G-50 gel filtration chromatography. | ||
At the same time, increased A430/A280 and A482/A280 purity ratios for PPC could be observed. Through two-step chromatography, the relative absorbance of A430/A280 increased almost 3 fold, and the content of chlorophyll was determined to be 0.94 g g−1 protein by spectrophotometry (Table 1). There was also a 2.6 fold increase in purity ratio of A482/A280 through the isolation procedure. The major carotenoid existing in the fraction was confirmed to be lutein by HPLC and was quantified to be 1.53 g g−1 protein for PPC (Table 2).
| Chlorophyll (g g−1 protein) | Purity ratio (A430/A280) | |
|---|---|---|
| Crude extract | 0.40 | 1.15 |
| PPC | 0.94 | 3.17 |
| Lutein (g g−1 protein) | Purity ratio (A482/A280) | |
|---|---|---|
| Crude extract | 0.69 | 0.37 |
| PPC | 1.53 | 0.95 |
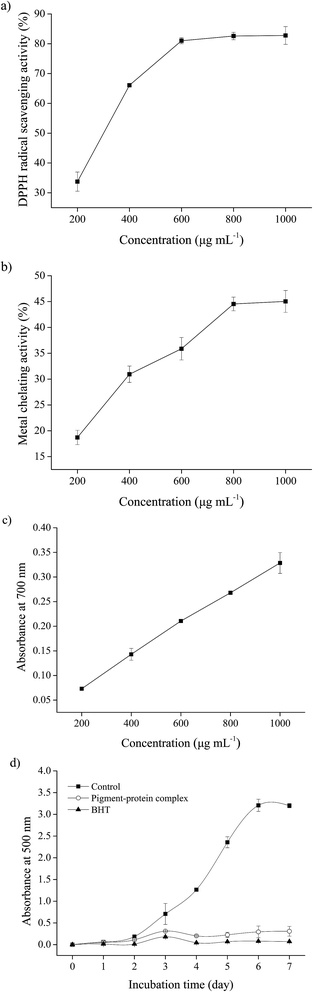
3.2. Antioxidant activity of the pigment–protein complex in vitro
To evaluate the antioxidant activity of the pigment–protein complex from Chlorella vulgaris, different antioxidant parameters were determined in vitro.3.3. Effect on CCl4-induced hepatic injury in mice
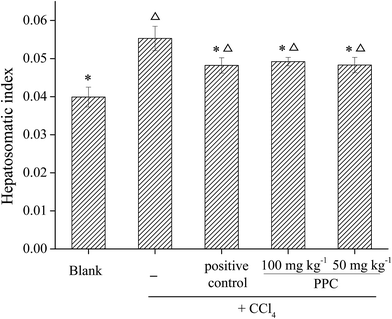
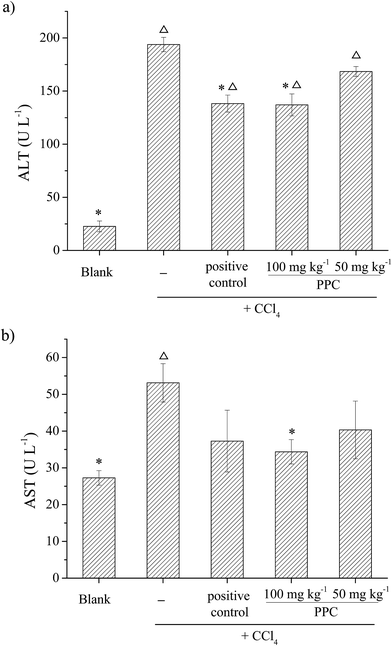
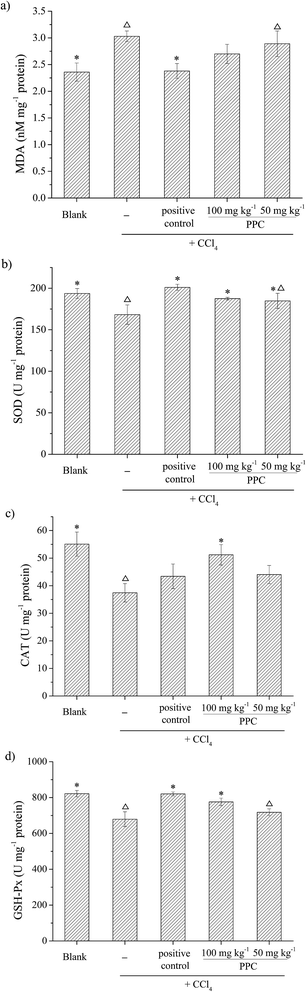
CCl4 is a widely known hepatotoxic revulsant, the metabolism of which results in the rapid generation of reactive radicals such as the trichloromethyl peroxyl radical (CCl3O3˙) and then initiates lipid peroxidation, leading to cell membrane damage, intracellular enzyme leakage and even cell necrosis. It has been demonstrated that hepatoprotective effects may be associated with antioxidant capacity.29 The natural pigment, carotenoid lutein, which has been verified to have significant hepatoprotective effects in CCl4-induced liver damage, was used at 20 mg kg−1 BW as a positive control.30Antioxidant enzymes such as SOD, CAT and GSH-Px act as the first line of defense against free radical induced oxidative stress. SOD is an effective defense enzyme that catalyzes the reduction of super anions into H2O2 and O2, and H2O2 can be further converted into H2O and O2 by CAT and GSH-Px.32 The status of these enzymes is an appropriate indirect assessment of the pro-oxidant-antioxidant status in tissues.29 The results showed that the activities of SOD, CAT and GSH-Px were remarkably decreased after exposure to CCl4 by 13.1%, 32.1% and 17.3% respectively (Fig. 6b–d), indicating oxidative damage to the liver. However, the suppressed enzyme activities could be restored by PPC pretreatment and the effects were significant at a concentration of 100 mg kg−1 BW (p < 0.05), implying that the hepatoprotective effects of PPC in CCl4-induced liver damage result from the stabilization of intracellular antioxidant defense systems.
The antioxidant activity of an antioxidant compound has been attributed to various mechanisms, among which are radical scavenging, binding of transition metal ion catalysts, reductive capacity, prevention of chain reaction initiation, decomposition of peroxides and prevention of continued hydrogen abstraction.27 The results obtained from this study clearly indicate that PPC had powerful antioxidant activity via various antioxidant mechanisms in vitro. Protein provided the pigments with good stability and solubility in aqueous solution, and the hepatoprotective effects of PPC might be attributed to the various antioxidant effects of the pigments.
4. Conclusions
The antioxidant and hepatoprotective activities of pigments in the form of a pigment–protein complex were investigated in the present study. The results showed that the natural pigment–protein complex isolated from Chlorella vulgaris exhibited significant antioxidant activities in vitro, including DPPH radical scavenging, metal chelation, reducing capacity and lipid peroxidation inhibition. Moreover, PPC manifested discernible protective action in CCl4-induced hepatotoxicity in vivo, indicating that PPC could be a promising candidate for use in dietary supplements against free radical-induced damage.Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 31571779), High & New project of Fujian Marine Fisheries Department (No. [2015]20), the National Marine Public Welfare Project (No. 201305022), Regional Demonstration of Marine Economy Innovative Development Project (No. 12PYY001SF08).References
- E. Mendis, N. Rajapakse and S. K. Kim, J. Agric. Food Chem., 2005, 53, 581–587 CrossRef CAS PubMed.
- L. Li, W. Li, Y. H. Kim and Y. W. Lee, Exp. Toxicol. Pathol., 2013, 65, 73–80 CrossRef CAS PubMed.
- R. L. Prior, L. W. Gu, X. L. Wu, R. A. Jacob, G. Sotoudeh, A. A. Kader and R. A. Cook, J. Am. Coll. Nutr., 2007, 26, 170–181 CrossRef CAS PubMed.
- D. Correa-Llantén, M. Amenábar and J. Blamey, J. Microbiol., 2012, 50, 374–379 CrossRef PubMed.
- P. Kuczynska, M. Jemiola-Rzeminska and K. Strzalka, Mar. Drugs, 2015, 13, 5847–5881 CrossRef PubMed.
- M. Zubia, Y. Freile-Pelegrín and D. Robledo, J. Appl. Phycol., 2014, 26, 2001–2010 CrossRef CAS.
- G. W. Burton and K. U. Ingold, Science, 1984, 224, 569–573 CAS.
- E. R. Sindhu, K. C. Preethi and R. Kuttan, Indian J. Exp. Biol., 2010, 48, 843–848 CAS.
- Y. Endo, R. Usuki and T. Kaneda, J. Am. Oil Chem. Soc., 1985, 62, 1387–1390 CrossRef CAS.
- C. Y. Hsu, P. Y. Chao, S. P. Hu and C. M. Yang, Food Nutr. Sci., 2013, 4, 1–8 CrossRef CAS.
- R. Halim and M. Danquah, in Advanced Biofuels and Bioproducts, ed. J. W. Lee, Springer, New York, 2013, pp. 807–832 Search PubMed.
- M. Vishnevetsky, M. Ovadis and A. Vainstein, Trends Plant Sci., 1999, 4, 232–235 CrossRef PubMed.
- H. van Amerongen and R. Croce, Photosynth. Res., 2013, 116, 251–263 CrossRef CAS PubMed.
- R. Fujii, M. Kita, Y. Iinuma, N. Oka, Y. Takaesu, T. Taira, M. Iha, R. J. Cogdell and H. Hashimoto, Photosynth. Res., 2012, 111, 157–163 CrossRef CAS PubMed.
- X. C. Qin, M. Suga, T. Y. Kuang and J. R. Shen, Science, 2015, 348, 989–995 CrossRef CAS PubMed.
- H. Ye, K. Wang, C. Zhou, J. Liu and X. Zeng, Food Chem., 2008, 111, 428–432 CrossRef CAS PubMed.
- Z. D. Hao, Y. Y. Liu, X. G. Xu, Q. Liu and P. H. Liu, Sci. Technol. Food Ind., 2010, 31, 369–372 CAS.
- D. I. Arnon, Plant Physiol., 1949, 24, 1–15 CrossRef CAS PubMed.
- D. P. Maxwell, S. Falk and N. P. A. Huner, Plant Physiol., 1995, 107, 687–694 CAS.
- H. C. Wu, H. M. Chen and C. Y. Shiau, Food Res. Int., 2003, 36, 949–957 CrossRef CAS.
- T. C. Dinis, V. M. Madeira and L. M. Almeida, Arch. Biochem. Biophys., 1994, 315, 161–169 CrossRef CAS PubMed.
- M. Oyaizu, Nippon Shokuhin Kogyo Gakkaishi, 1988, 35, 771–775 CrossRef CAS.
- T. Osawa and M. Namiki, Agric. Biol. Chem., 1981, 45, 735–739 CrossRef CAS.
- H. Mitsuda, K. Yuasumoto and K. Iwami, Eiyo to Shokuryo, 1966, 19, 210–214 CrossRef CAS.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- O. P. Sharma and T. K. Bhat, Food Chem., 2009, 113, 1202–1205 CrossRef CAS.
- I. Gülcin, H. A. Alici and M. Cesur, Chem. Pharm. Bull., 2005, 53, 281–285 CrossRef.
- I. Andreu, D. Neshchadin, E. Rico, M. Griesser, A. Samadi, I. M. Morera, G. Gescheidt and M. A. Miranda, Chemistry, 2011, 17, 10089–10096 CrossRef CAS PubMed.
- Q. Liu, G. Tian, H. Yan, X. Geng, Q. Cao, H. Wang and T. B. Ng, J. Agric. Food Chem., 2014, 62, 8858–8866 CrossRef CAS PubMed.
- L. P. Pei, Chin. J. Gerontol., 2009, 29, 685–687 CAS.
- A. Vanitha, K. N. Murthy, V. Kumar, G. Sakthivelu, J. M. Veigas, P. Saibaba and G. A. Ravishankar, Int. J. Toxicol., 2007, 26, 159–167 CrossRef CAS PubMed.
- A. Pareek, A. Godavarthi, R. Issarani and B. P. Nagori, J. Ethnopharmacol., 2013, 150, 973–981 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |