Synthesis and characterization of novel electrochromic poly(amide-imide)s with N,N′-di(4-methoxyphenyl)-N,N′-diphenyl-p-phenylenediamine units†
Abstract
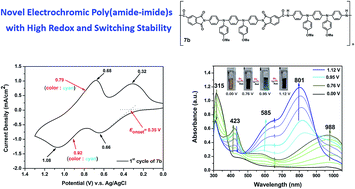
We developed a new efficient procedure for the synthesis of a bistriphenylamine diamine monomer, N,N′-bis(4-aminophenyl)-N,N′-bis(4-methoxyphenyl)-1,4-phenylenediamine (3). A new dicarboxylic acid monomer bearing two built-in imide rings, namely N,N′-bis(trimellitimidophenyl)-N,N′-bis(4-methoxyphenyl)-1,4-phenylenediamine (4), was synthesized from the condensation of diamine 3 with two equivalent amounts of trimellitic anhydride. Several novel electroactive poly(amide-imide)s (PAIs) containing N,N′-di(4-methoxyphenyl)-N,N′-diphenyl-p-phenylenediamine [TPPA(OMe)2] units have been prepared by the phosphorylation polyamidation reactions from diamine 3 with four imide ring-preformed dicarboxylic acids or from diimide-diacid 4 with 4,4′-oxydianiline and diamine 3, respectively. All the PAIs were readily soluble in many organic solvents and could be solution-cast into tough and flexible polymer films. These PAIs exhibited glass-transition temperatures (Tgs) in the range 206–292 °C, and most of them did not show significant weight-loss before 450 °C. The PAI films revealed reversible electrochemical oxidation processes accompanied with strong color changes from the pale yellow neutral state to yellowish green and deep blue oxidized. Incorporating the TPPA(OMe)2 unit on the amide side of PAIs led to lower oxidation potentials and higher redox and electrochromic stability.

 Please wait while we load your content...
Please wait while we load your content...