Effect of Pb2+, Cd2+, Cu2+ and dissolved organic carbon (DOC) on the distribution and partition of decabromodiphenyl ether (BDE-209) in a water–sediment system†
Donggao Yina,
Hui Pengb,
Hua Yin*a,
Su Zhoua,
Shichang Xiongc,
Zehua Liua and
Zhi Danga
aKey Laboratory of Ministry of Education on Pollution Control and Ecosystem Restoration in Industry Clusters, School of Environment and Energy, South China University of Technology, Guangzhou 510006, P. R. China. E-mail: huayin@scut.edu.cn; Tel: +86 20 39380569
bDepartment of Chemistry, Jinan University, Guangzhou 510632, P. R. China
cDepartment of Environmental Engineering, Jinan University, Guangzhou 510632, P. R. China
First published on 23rd November 2015
Abstract
The combined pollution of polybrominated diphenyl ethers (PBDEs) and heavy metals in electronic waste dismantling areas has received increasing concern in recent years. The distribution and partition of decabromodiphenyl ether (BDE209, a major constituent in the PBDE family) in a water–sediment system in the presence of Pb2+, Cd2+ and Cu2+ at different concentrations were systematically investigated in this study, with the goal of revealing the adsorption behavior of BDE209 co-existent with heavy metals in sediment. The experimental results revealed that all the metals increased the sorption capacity of BDE209 of the sediment (p < 0.05) in the order of Pb2+ > Cd2+ > Cu2+, and the concentration effects of Pb2+ and Cd2+ were obvious compared with Cu2+. A linear equation fitted well with the adsorption process, and the partition coefficient Kd increased with increasing concentrations of the heavy metals in the range of 0–10 mg L−1. Dissolved organic carbon (DOC) played a vital role in BDE209 sorption. When co-existent with heavy metals, DOC, particularly larger-sized DOC molecules in liquid phase decreased significantly and tended to migrate to the sediment. This modification led to a significant increase in the capacity of BDE209 sorption by the sediment. In addition, heavy metals decreased the stability of the water–sediment system, and the stability of the system decreased gradually with the increase of heavy metal concentration, as a result of combination with DOC. These combined to increase the BDE209 sorption by the sediment.
1. Introduction
As a class of commonly used fire retardants,1 polybrominated diphenyl ethers (PBDEs) were widely employed in plastic products, textiles, electronic products, building materials, etc.2 Because of a lack of chemical bond strength with other molecules, PBDEs were prone to releasing and being introduced to all kinds of environmental media including soil, atmosphere, water bodies and organisms.3,4 Among these, water bodies acted as both important sinks and carriers of PBDEs.5 Due to weak water-solubility and strong fat-solubility, PBDEs were easily adsorbed by sediment and suspensions when they entered into water bodies.6 The adsorption by sediment plays a vital role in the transformation and bioavailability of toxic organic compounds such as PBDEs, polynuclear aromatic hydrocarbons (PAHs), pesticides, etc., in aquatic environments.7,8Dissolved organic carbon (DOC) is an important constituent of sediment. Because of its strong chelating and adsorbing abilities, DOC can exert significant influence on the mobility, transformation and biodegradation of organic pollutants.9,10 Close attention has been paid to DOC in research on the fate of PBDEs in water–sediment systems in recent years.11 Luo, et al.12 found that DOC and particulate organic carbon (POC) could play certain roles in determining the distribution and partition of PBDEs between particles and dissolved phases. It was also reported that POC and DOC were the major factors that influenced the partitioning and fate of hydrophobic contaminants in a coastal environment.13
Heavy metal contamination is notorious for its universality in the environment and its strong toxicity to organisms.14,15 It was reported that different kinds of heavy metals such as Cu2+, Pb2+ and Cd2+ were discharged into the environment when dismantling electronic waste using primitive methods, in some places in the world, including China.16 These heavy metals coexisted with hydrophobic contaminants and posed severe ecological risks, which made the bioremediation of polluted water and soil more difficult to conduct.17,18 To deal with this challenge, it was worth considering the partition and transformation of hydrophobic contaminants in the water–sediment system under the effect of heavy metals. It has been reported that copper-influenced sorption of diethyl phthalate (DEP) and di-n-butyl phthalate (DnBP) was due to the binding of copper to DOC that led to the configuration change of DOC and thus to its enhanced sorption to sediment.19 However, the information regarding the influence of heavy metals on the partition of PBDEs is severely limited thus far.
In this study, we chose decabromodiphenyl ether (BDE209), the most mass-produced and widely used fire retardant among the PBDE family,20 as a model compound to investigate the adsorption capacity of PBDEs of sediment and the effect of heavy metals on the sorption behavior. The sorption mechanism was further discussed in terms of sediment constituents, DOC morphology and the zeta potential of the adsorption system.
2. Material and methods
2.1 Chemicals
BDE209 (purity 98%) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Organic solvents including n-hexane, dichloromethane, acetone and toluene were chromatographically pure, and purchased from Sigma.Analytical-grade salts of Pb(NO3)2, Cd(NO3)2 and Cu(NO3)2 were obtained from Guangzhou Chemical Reagent Company, China. All other chemicals were of reagent quality.
2.2 Sediment sampling
Sediment was collected from the countryside of Shantou city, far away from urban and industrial areas, in Guangdong province, China. The top 5 cm layer of sediment was collected, wrapped in tinfoil and enclosed in zippered bags to avoid loss. After being transported to the laboratory, the sample was air dried at room-temperature. Then the dry sediment was ground and sieved to collect a fraction less than 100 mesh. The homogenized sediment was protected from light and samples selected by quartering. There was no detected BDE209 in sediment samples. The test results revealed that the concentration of inherent BDE209 in sediment was very low and that of heavy metals was within normal natural background values, thus their influence was negligible.The physicochemical properties of the sediment were as follows: the main components (dry weight) of the sediment were 13% clay, 14% silt and 73% sand, respectively; the soil organic carbon content (foc) was 3.4%; the cation exchange capacity (CEC) was 9.86 cmol(+)/kg; the Fe and Mn content was 197 and 9.2 μmol g−1, respectively; and the pH value (sediment![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 1
water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 (w/v)) was 6.8.
2.5 (w/v)) was 6.8.
2.3 BDE209 extraction and analysis
2.4 Sorption experiments
Batch experiments were conducted to investigate the sorption isotherms and the effect of metal ions on the BDE209 sorption by sediment. A sediment of 0.5 g was mixed with 10 mL deionized water containing different concentrations of heavy metals (0, 2, 3, 5 and 10 mg L−1) and BDE209 (0.104, 0.208, 0.312, 0.52, 1.04 μmol L−1) in a 50 mL glass tube with a cap. The tube was agitated at 30 °C for 48 h in a rotary shaker at 220 rpm to ensure sorption equilibrium. To determine the effect of the metal ions (Pb2+, Cd2+, Cu2+) on the partition of BDE209 in the water–sediment system, the initial concentrations of Pb2+, Cd2+ and Cu2+ were adjusted to four levels of 2, 3, 5 and 10 mg L−1, respectively. A sample without an addition of metals served as a control. The other experimental procedures were the same as described above.After reaching the sorption equilibrium, the BDE209 content in both the aqueous and solid phase was determined according to the methods described in Section 2.3. All of the experiments were conducted in triplicate and repeated twice to check the reproducibility of the results.
2.5 Physicochemical characterization of aqueous/solid phases
The adsorption experiments were carried out according to Section 2.4. After the adsorption equilibrium was achieved, aqueous and solid phases were separated by centrifugation. The dissolved organic carbon (DOC) in solution was determined by a total organic carbon (TOC) analyzer (Shimadzu TOC-Vcsh, Japan). The supernatant was air dried and re-dissolved in 1 mL heavy water for analysis by nuclear magnetic resonance (NMR) (Optima 600 MHz, USA) to further explore the constituents of the DOC. High performance size exclusion chromatography (HPSEC) (Waters, USA) was also employed to measure the molecular weight and dimension of DOC. The zeta potential of the adsorption system was detected after the system was adjusted to pH 6.0.3. Results and discussion
3.1 Effects of heavy metals on the sorption of BDE209 by sediment
The effect of Cd2+, Pb2+ and Cu2+ on the sorption of BDE209 (0.104 μmol L−1) by sediment is shown in Fig. 1. It was clear that all the metals increased the sorption capacity of BDE209 of sediment (p < 0.05) and residues of BDE209 in the liquid phase decreased continuously with increasing concentrations of the heavy metals. For the sorption system containing 2 mg L−1 Cd2+, the residues of BDE209 were 0.009 ± 0.001 μmol L−1; far below the control (0.011 ± 0.001 μmol L−1) when heavy metals were absent. The sorption capacity was reinforced with increasing Cd2+ concentration and was strongest at 10 mg L−1 Cd2+, with 0.007 ± 0.0002 μmol L−1 BDE209 left in the liquid phase. By comparison, the influence of the heavy metals was in the order of Pb2+ > Cd2+ > Cu2+. In short, when sediment was contaminated with heavy metals, it exhibited a stronger sorption capacity for BDE209, which might further reduce the bioavailability of BDE209,21 resulting in more difficulty in removing BDE209 from a water body.3.2 Sorption isotherms of BDE209 of sediment
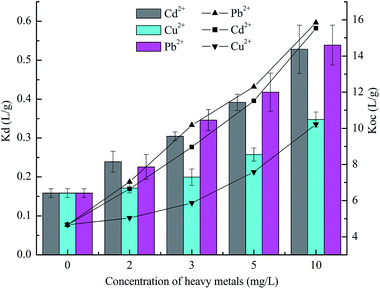
The sorption of BDE209 (0.104, 0.208, 0.312, 0.520 and 1.040 μmol L−1) by the tested sediments was conducted in the presence of heavy metals in the range of 0 to 10 mg L−1. After 48 h of agitation, the partition of BDE209 in water and sediment approached equilibrium. The final concentrations of BDE209 in the liquid and solid phases were determined and the correlation between them was simulated by isotherms to probe into the distribution mechanism of BDE209 in the phases. In all cases, the sorption could be well described by the linear isotherm with a correlation constant (R2) greater than 0.96 (see Table S1 in ESI†). In the presence of heavy metals, the partition coefficient Kd increased as the concentration of metals rose (shown in Fig. 2). That is, Cd2+, Pb2+, and Cu2+ had a positive effect on the sorption of BDE209 by sediment. It was also found that different heavy metals at the same concentration showed different degrees of ability for facilitating the sorption of BDE209 by the sediment, which was similar to the results obtained in Section 3.1. In the case of 10 mg L−1 heavy metal, the sorption capacity was enhanced by the heavy metals in the order of Pb2+ > Cd2+ > Cu2+ with a Kd of 0.637 ± 0.062, 0.623 ± 0.051 and 0.466 ± 0.019, respectively.The ideal linear simulation results revealed that partition played a vital role in BDE209 transport in the water–sediment system. The relationship between the BDE209 partition coefficient and heavy metal concentration was plotted in Fig. 3, in which Koc was the carbon-normalized partition coefficient,25
 | (1) |
3.3 Effect of heavy metals on DOC
It was reported that the adsorption of hydrophobic organic compounds by sediment was related to DOC and organic matter.23,24 In this experiment, DOC, characterized by total organic carbon (TOC) in supernatant, in a water–sediment system containing different concentrations of heavy metals was measured. DOC concentrations decreased with the increase of heavy metal concentration and the extent of influence of these heavy metals was in the order of Pb2+ > Cd2+ > Cu2+ (Fig. 4). This result corresponded with the alteration of the partition coefficient (Kd) (see Table S1 in ESI†). It indicated that heavy metals inhibited DOC from further dissolution in the liquid phase and thus DOC would be retained more in the solid phase. This is due to the fact that heavy metals could form complexes with organics and therefore the solubility of these organics is then reduced.25 As a kind of organic, DOC has strong affinity with organic pollutants,26 thus enhancing the sorption capacity of sediment for BDE209.According to the partition theory, a partition coefficient has positive correlation with the organic content.27 To further estimate the adsorption capacity of DOC for BDE209, the assumption is made that the increased organics in sediment, (i.e., DOC) have the same adsorption characterization as that of undissolved organics in sediment. The equations were expressed as follows:
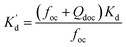 | (2) |
This equation could also be expressed as:
 | (3) |
| Heavy metals | Concentration (mg L−1) | Qdoc (mg kg−1) | Qdoc/foc (%) | ΔK′d/ΔKd |
|---|---|---|---|---|
| Cd2+ | 0 | 0 | 0 | — |
| 2 | 333.10 (10.80) | 0.98 (0.03) | 30.33 (0.90) | |
| 3 | 450.60 (14.30) | 1.32 (0.04) | 26.24 (0.77) | |
| 5 | 611.60 (18.90) | 1.80 (0.06) | 9.56 (0.31) | |
| 10 | 831.30 (19.60) | 2.44 (0.06) | 9.16 (0.22) | |
| Pb2+ | 0 | 0 | 0 | — |
| 2 | 382.60 (9.60) | 1.12 (0.03) | 29.89 (0.78) | |
| 3 | 506.30 (12.10) | 1.49 (0.04) | 14.43 (0.38) | |
| 5 | 659.40 (16.00) | 1.94 (0.05) | 11.45 (0.29) | |
| 10 | 832.60 (15.60) | 2.45 (0.05) | 10.54 (0.21) | |
| Cu2+ | 0 | 0 | 0 | — |
| 2 | 140.40 (7.80) | 0.41 (0.02) | 17.82 (0.83) | |
| 3 | 213.40 (16.50) | 0.63 (0.05) | 22.51 (1.66) | |
| 5 | 350.20 (18.00) | 1.03 (0.05) | 21.96 (1.02) | |
| 10 | 492.20 (9.90) | 1.45 (0.03) | 17.83 (0.36) |
3.4 NMR analysis of DOC in the supernatant
NMR analysis was carried out to further investigate the mechanism by which heavy metals increase the sorption capacity of BDE209 of the sediment. As seen from the NMR spectra in Fig. 5, the main functional group compositions of DOC in the supernatant included aliphatic moieties (at 0.5–2.8 ppm regions), alkyl moieties, and a combination of sugar, amino acid, aromatic methoxyl and CH2 units adjacent to ester groups (3.0–4.0 ppm regions). Compared to the control, the peak strength of DOC weakened when heavy metals co-existed, especially under the influence of Pb2+ and Cd2+, suggesting that heavy metals restrained the release of DOC to the aqueous phase, resulting in an obvious decrease of DOC in the supernatant, as evidenced in Fig. 4. This contributed to the high aliphatic moiety content of the DOC that favored its binding with fat soluble BDE209,29,30 leading to the promotion of BDE209 adsorption in the sediment. | ||
| Fig. 5 NMR spectra of DOC in supernatant. (CK) without heavy metals, (a) 10 mg L−1 Cu2+, (b) 10 mg L−1 Cd2+, (c) 10 mg L−1 Pb2+. | ||
3.5 HPSEC analysis of DOC in the supernatant
HPSEC was employed to evaluate the relative molecular size and distribution of DOC.31 HPSEC separates molecule particles based on their size. The larger sized fraction with higher molecular weight would pass through the chromatographic column faster, leading to a shorter retention time. As shown in Fig. 6, the release of larger DOC molecules (retention time 40–46 min) was restrained when heavy metals existed, since all the absorbance values with heavy metals were lowered, which was in proportion to the concentration of DOC. The decrease of higher molecular weight DOC under the influence of heavy metals was in the order of Pb2+ > Cd2+ > Cu2+, which indicated that heavy metals were liable to complex with larger DOC particles and sequester them from the aqueous phase. As for the smaller DOC molecules (retention time 46–52 min), Pb2+, Cd2+ and Cu2+ exhibited different effects. In the Pb2+ containing system, small molecular DOC decreased significantly, while no obvious variation was presented in the Cu2+ and Cd2+ containing systems, compared with the control, which also agreed well with our previous adsorption experiments.3.6 Zeta potential determination of the supernatant
The value of the zeta potential is related to the stability of colloidal dispersions. Colloids with high zeta potential (negative or positive) are electrically stabilized while those with low zeta potential tend to coagulate or flocculate.32 In this experiment, the zeta potential of the BDE209 sorption system was measured and the result is presented in Fig. 7. It was evident that when heavy metals were added into the system, the absolute value of the zeta potential in the supernatant was significantly reduced with the rise of metal concentration, suggesting that heavy metals enhanced the aggregation of negatively charged DOC molecules, which was positively correlated with the stability of the system. Also, heavy metals weakened the stability of the system in the order of Pb2+ > Cd2+ > Cu2+, which was consistent with the result obtained in Section 3.5. On the one hand, the added heavy metals combined with DOC molecules, especially the larger size ones in the sediment, resulting in more of these molecules being kept in the sediment, and leading to the promotion of BDE209 sorption by the sediment. On the other hand, the combination of heavy metals and DOC gave rise to an easier adsorption to occur in the sediment, which not only increased the adsorption capacity of BDE209, but also reduced the mobility of the heavy metals and BDE209. These two mechanisms are both involved in the adsorption of BDE209 in the presence of heavy metals.4. Conclusions
Heavy metals Cu2+, Cd2+ and Pb2+ advanced the sorption of BDE209 by sediment and the strength of this effect was in the order of Pb2+ > Cd2+ > Cu2+. Over a range of experimental conditions, heavy metals in higher concentrations brought about a stronger adsorption capacity. The sorption isotherms of BDE209 in sediment fitted well with a linear distribution-type model (R2 > 0.96). BDE209 sorption by sediment was relative to DOC in sediment. Lipids are one of the main classes of components in DOC, which made a significant contribution to BDE209 adsorption. When coexistent with heavy metals, DOC, especially the larger-sized molecules were liable to form complexes with heavy metals (Cu2+, Cd2+ and Pb2+) and consequently resulted in migration to the sediment. Furthermore, heavy metals weakened the colloidal stability of the water–sediment system, leading to an increase in the adsorption capacity of BDE209 and a decrease in the migration capability of heavy metals and BDE209.Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (No. U1501234, U0933002, 41330639), Natural Science Foundation of Guangdong Province, China (S2013020012808), and the Fundamental Research Funds for the Central Universities (No. 2015ZP027) for the financial support of this work.References
- M. Lei, N. Wang, L. H. Zhu, C. S. Xie and H. Q. Tang, Chem. Eng. J., 2014, 241, 207–215 CrossRef CAS.
- C. H. Xia, J. C. W. Lam, X. G. Wu, L. G. Sun, Z. Q. Xie and P. K. S. Lam, Chemosphere, 2011, 82, 18–24 CrossRef CAS PubMed.
- R. Lavandier, N. Quinete, R. A. Hauser-Davis, P. S. Dias, S. Taniguchi, R. Montone and I. Moreira, Chemosphere, 2013, 90, 2435–2443 CrossRef CAS PubMed.
- J. G. Allen, M. D. Mcclean, H. M. Stapleton, J. W. Nelson and T. F. Webster, Environ. Sci. Technol., 2007, 41, 4574–4579 CrossRef CAS PubMed.
- A. Möller, Z. Y. Xie, R. Sturm and R. Ebinghaus, Environ. Pollut., 2011, 159, 1577–1583 CrossRef PubMed.
- S. Lee, K. Kannan and H. B. Moon, Sci. Total Environ., 2013, 443, 24–30 CrossRef CAS PubMed.
- B. Chefetz and B. S. Xing, Environ. Sci. Technol., 2009, 43, 1680–1688 CrossRef CAS PubMed.
- B. Q. Zhu, J. C. W. Lam, S. Y. Yang and P. K. S. Lam, Chemosphere, 2013, 93, 555–560 CrossRef CAS PubMed.
- M. L. Wei-Haas, K. J. Hageman and Y. P. Chin, Environ. Sci. Technol., 2014, 48, 4852–4859 CrossRef CAS.
- J. Akkanen, R. D. Vogt and J. V. K. Kukkonen, Aquat. Sci., 2004, 66, 171–177 CrossRef CAS.
- L. Delgado-Moreno, L. Wu and J. Gan, Environ. Sci. Technol., 2010, 44, 8473–8478 CrossRef CAS PubMed.
- X. J. Luo, M. Yu, B. X. Mai and S. J. Chen, Chin. Sci. Bull., 2008, 53, 493–500 CrossRef CAS.
- M. Kuivikko, K. Sorsa, J. V. K. Kukkonen, J. Akkanen, T. Kotiaho and A. V. Vahatalo, Environ. Toxicol. Chem., 2010, 29, 2443–2449 CrossRef CAS PubMed.
- E. Dimitrakakis, A. Janz, B. Bilitewski and E. Gidarakos, Waste Manag., 2009, 29, 2700–2706 CrossRef CAS PubMed.
- H. Yin, B. Y. He, X. Y. Lu, H. Peng, J. S. Ye and F. Yang, Water Res., 2008, 42, 3981–3989 CrossRef CAS.
- H. M. Wang, M. Han, S. W. Yang, Y. Q. Chen, Q. A. Liu and S. Ke, Environ. Int., 2011, 37, 80–85 CrossRef CAS PubMed.
- G. Y. Shi, H. Yin, J. S. Ye, H. Peng, J. Li and C. L. Luo, J. Hazard. Mater., 2013, 263, 711–717 CrossRef CAS PubMed.
- W. Zhang, M. Zhang, S. An, K. F. Lin, H. Li, C. Z. Cui, R. B. Fu and J. Zhu, Environ. Toxicol. Pharmacol., 2012, 34, 358–369 CrossRef CAS PubMed.
- N. Xu, J. R. Ni, W. L. Sun and A. G. L. Borthwick, Chemosphere, 2007, 69, 1419–1427 CrossRef CAS PubMed.
- H. Fromme, W. Körner, N. Shahin, A. Wanner, M. Albrecht, S. Boehmer, H. Parlar, R. Mayer, B. Liebl and G. Bolte, Environ. Int., 2009, 35, 1125–1135 CrossRef CAS PubMed.
- S. L. Klosterhaus, E. Dreis and J. E. Baker, Environ. Toxicol. Chem., 2011, 30, 1204–1212 CrossRef CAS PubMed.
- Z. Y. Zhang, K. Sun, B. Gao, G. X. Zhang, X. T. Liu and Y. Zhao, J. Hazard. Mater., 2011, 190, 856–862 CrossRef CAS PubMed.
- J. P. Gao, J. Maguhn, P. Spitzauer and A. Kettrup, Water Res., 1998, 32, 2089–2094 CrossRef CAS.
- L. Delgado-Moreno, L. Wu and J. Gan, Environ. Sci. Technol., 2010, 44, 8473–8478 CrossRef CAS PubMed.
- Y. Z. Gao, W. Xiong, W. T. Ling and J. M. Xu, Chemosphere, 2006, 65, 1355–1361 CrossRef CAS PubMed.
- H. Choi and S. R. Al-Abed, J. Hazard. Mater., 2009, 165, 860–866 CrossRef CAS.
- L. Luo, S. Z. Zhang, Y. B. Ma, P. Christie and H. L. Huang, Environ. Sci. Technol., 2008, 42, 2414–2419 CrossRef CAS.
- M. Kuivikko, K. Sorsa, J. V. K. Kukkonen, J. Akkanen, T. Kotiaho and A. V. Vahatalo, Environ. Toxicol. Chem., 2010, 29, 2443–2449 CrossRef CAS PubMed.
- M. J. He, X. J. Luo, M. Y. Chen, Y. X. Sun, S. J. Chen and B. X. Mai, Sci. Total Environ., 2012, 419, 109–115 CrossRef CAS.
- Y. M. Chen, M. Yu, X. J. Luo, S. J. Chen and B. X. Mai, Mar. Pollut. Bull., 2011, 62, 29–35 CrossRef PubMed.
- S. Simon, B. Païro, M. Villain, P. D’Abzac, E. Van Hullebuschb, P. Lens and G. Guibaud, Bioresour. Technol., 2009, 100, 6258–6268 CrossRef CAS PubMed.
- O. M. Lage, J. Bondoso and J. A. M. Catita, Arch. Microbiol., 2012, 194, 847–855 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17443k |
| This journal is © The Royal Society of Chemistry 2015 |