Preparation of a reduced graphene oxide/zirconia nanocomposite and its application as a novel lubricant oil additive†
Abstract
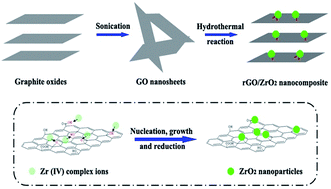
Nanocomposites consisting of zirconia (ZrO2) nanoparticles and reduced graphene oxide (rGO) nanosheets were successfully fabricated by a one-pot hydrothermal method. By regulating the proportion of the precursors of the GO colloidal suspension and zirconium oxychloride (ZrOCl2) solution, ZrO2 nanoparticles with a diameter of about 5 nm were uniformly anchored onto the rGO nanosheets. The combination mechanism of ZrO2 nanoparticles fully bonded onto rGO nanosheets is the formation of the monodentate or bidentate composites between the oxygen-containing groups of GO and Zr(IV) complex ions from hydrolysis of the ZrOCl2 solution. The dispersibility and tribological properties of the prepared composites were investigated as novel lubricant additives in paraffin oil. The results suggested that the oil with a small amount of nanocomposite (0.06 wt%) exhibits good dispersibility, excellent friction-reduction and anti-wear properties as well as a high load-bearing capacity caused by the synergistic effect of the rGO nanosheets and ZrO2 nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...