Novel phosphorescent iridium(III) complexes containing 2-thienyl quinazoline ligands: synthesis, photophysical properties and theoretical calculations†
Qunbo Mei‡
*a,
Jiena Weng‡ac,
Zhijie Xua,
Bihai Tongb,
Qingfang Huaa,
Yujie Shia,
Juan Songa and
Wei Huang*ac
aKey Laboratory for Organic Electronics and Information Displays, Institute of Advanced Materials(IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts & Telecommunications, 9 Wenyuan Road, Nanjing 210023, China. E-mail: iamqbmei@njupt.edu.cn
bCollege of Metallurgy and Resources, Anhui University of Technology, Ma'anshan, Anhui 243002, P. R. China
cKey Laboratory of Flexible Electronics (KLOFE), Institute of Advanced Materials(IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China. E-mail: iamwhuang@njupt.edu.cn
First published on 9th November 2015
Abstract
The easy tailoring of organic ligands of iridium(III) complexes provides a facile way to tune their opto-electronic properties for applications in high efficiency phosphorescent light emitting diodes. Herein, a series of yellow and red emitting phosphorescent iridium complexes based on 2-thienyl quinazoline derivatives are successfully synthesized and systematically characterized with various opto-electronic properties. The X-ray crystal structures demonstrate that the iridium centers in the complexes with bulky substituents on the 4-position of quinazolyl rings prefer to chelate with the N atoms in the 1-position of quinazolyl rings. Both experiment and theoretical studies indicate that the steric hindrance along with the electron-donating effect of substituents on the C^N ligands enhances the emission quantum yields, accompanied by significant emission shifts. Two yellow phosphorescent iridium complexes (Ir2 and Ir3) are successfully designed and exhibit moderate emission efficiencies, through the incorporation of bulky ligands with strong electron-donating abilities (piperidine for Ir2 and 2,6-dimethyl-phenoxy for Ir3, respectively). The synergistic effect of electron structure and hindrance of ligand is believed to be a promising strategy for tuning the opto-electronic properties of iridium complexes.
Introduction
Phosphorescent iridium(III) cyclometalated complexes have attracted great attention as emitting guest materials in organic light-emitting diodes (OLEDs) owing to their advantageous properties, such as high quantum efficiency, relatively short lifetime and excellent color purity.1,2 Nowadays, full visible-spectrum monochromatic phosphorescent OLEDs (PhOLEDs) can be easily realized by using iridium complexes as emissive phosphors. The external quantum efficiencies of the blue, green and red PhOLEDs have achieved or even exceeded 20%.3 Recently, yellow/orange phosphorescent iridium complexes have received a surge of interest in design of high performance two-color (blue and yellow/orange) and white OLEDs (WOLEDs) because of both in device efficiency and fabrication cost.2 Moreover, the yellow/orange phosphorescent iridium complexes are beneficial to improve the device efficiencies as well as the color rendering index for four-color (blue, green, yellow/orange and red) WOLEDs.4 However, it remains an urgent demand to realize desired power efficiency (exceed 100 lm W−1) at high luminance in yellow/orange PhOLEDs, which plays an important role in WOLEDs for practical lighting applications.2 Herein, the development of appropriate materials and elaborate device structures is still greatly demanded.Experimental and theoretical studies reported to date have established that the phosphorescence emission of iridium complexes can be easily tailored with various electron donating or withdrawing ligands.5,6 In particular, modification of cyclometalating ligand (C^N ligand), for example, ppy (2-phenylpridinato-C2, N), is able to tune the emission color over the entire visible region.5 Since the π* orbital of the pyridyl ring of the ppy ligand governs the lowest unoccupied molecular orbital (LUMO) level of the ppy based iridium complexes,7 adding electron-donating groups to the pyridyl rings is supposed to raise the LUMO level, which results in enlarged band gap and blue-shifted emission. It is noted that one promising way to increase the quantum efficiency of organic light emitting material is proposed as the steric hindrance approach, together with highly stability of spectra in device performance.8 According to our previous work,9 the steric hindrance effect of spirofluorene can suppress the aggregation of conjugated polymer chain leading to high efficiency and stable blue emission. Recently, we further proposed a supramolecular steric hindrance approach with spirofluorene based iridium complex that exhibited impressive quantum yields and spectral stability.10 Therefore, it is reasonable to utilize the synergistic effect of electron structure and hindrance of ligand to design high efficient yellow/orange iridium complexes by introducing of appropriate electron-donating bulky substituent into the quinazolinyl ring.11
In this work, four novel yellow/orange iridium complexes Ir1–Ir4 are reported, which consist of 2-thienyl quinazoline (TQZ) derivative as the C^N ligands and a picolinate (pic) as the ancillary N^O ligand (Scheme 1). Diamine (piperidine (PD) and diphenylamine (DPA)) and 2,6-dimethyl-phenoxy (DMP) are introduced as electron-donating groups at the 4 position of the quinazolinyl group to tuning the emission color. The incorporation of PD and DMP is aim to enhance appropriate electron-donating effect without extending conjugation on the C^N ligand π-system. The time-depended density functional theory (TD-DFT) calculation based on the first triplet excited state (T1) optimized structures shows that both PD and DMP groups affect the LUMO level than the highest occupied molecular orbital (HOMO) level, resulting in a larger band gap and then leading the emission of Ir2 and Ir3 blue-shifted as compared to Ir1. Conversely, the incorporation of DPA substituent is capable to improve the electron-donating effect but increase the π-conjugated system on the C^N ligand, which change the band gap slightly. In addition, all these substituents can provide steric hindrance around the metal to increase the photoluminescence quantum yields (PLQYs).
Results and discussion
Synthesis
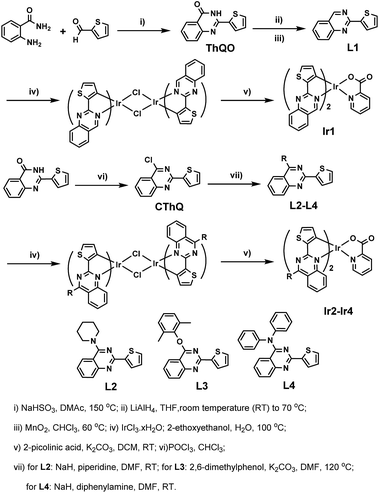
The synthetic procedures of the cyclometalated ligands L1–L4 and the relevant complexes Ir1–Ir4 are depicted in Scheme 2. Every step of the reactions sequence proceeded efficiently to give good or moderate yield of the target products. The cyclometalated ligand L2 and L4 are conveniently synthesized by N-alkylation of piperidine or diphenylamine with 4-chloro-2-(thiophen-2-yl)quinazoline in the presence of NaH in a polar solvent according to the literature procedures.12 Meanwhile, the ligand L3 is prepared with similar method by taking place of NaH with finely ground potassium carbonate. Since the chlorine atom on quinazolinyl ring can be functionalized easily, these reactions are proceeded in mild conditions and give satisfactory yields. And then, the iridium complexes are synthesized according to the Nonoyama's method.13 The classical dimeric iridium(III) intermediate (C^N)2Ir(μ-Cl)2Ir(C^N)2 are prepared by direct reaction of IrCl3·xH2O with Ln (n = 1–4). Subsequently, complexes Ir1–Ir4 are readily synthesized from the dimers and 2-picolinic acid in DCM solution by a conventional synthetic method, which are purified by column chromatography on aluminum oxide and isolated in good yield (31.4–61.7%).X-ray crystal structures
High-quality single crystals of complexes Ir1–Ir4 (ESI†) are obtained by slow diffusion of their tetrahydrofuran solution into isopropanol and characterized by X-ray crystallography (Fig. 1). The corresponding crystallographic data are collected in ESI.† All crystals exhibit distorted octahedral geometries around the iridium center with two C^N ligands and one N^O ligand. The two nitrogen atoms of C^N ligands exhibit cis-C, C and trans-N, N chelate dispositions. Selected bond lengths and angles are listed in Tables S2 and S3.† As shown in Fig. 1, while the iridium atom in the complex Ir1 chelates with N at the 3 position of the quinazolyl ring, the introduction of substituent significantly enhances the steric hindrance of the C^N ligands, leading the iridium atoms in the complexes Ir2–Ir4 chelate with N at the 1 position of the quinazolyl ring. Therefore, the Ir–N bonds around the iridium center of Ir2–Ir4 are slightly longer than that of Ir1, while the Ir–C bonds are slightly shorten. Additionally, the C–Ir–C angles of Ir2–Ir4 are in the range of 92.6° ∼ 95.3°, which are obviously enlarged comparing to that of Ir1 (86.9°).Photophysical properties
Effects of different electron-donating substituents on the photophysical and electrochemical properties have been investigated. The UV-vis absorption and photoluminescent (PL) spectra of Ir(III) complexes Ir1–Ir4 in dichloromethane (DCM) at room temperature are shown in Fig. 2 and the spectroscopic data collected from these spectra are summarized in Table 1. As most iridium complexes, the dominant absorption bands at 240–350 nm can be assigned to spin-allowed π–π* transitions from the C^N ligands. The lower-energy absorption bands, which extends up to 600 nm, can be attributed to a mixture of ligand-to-ligand charge transfer (LLCT, π(C^N) → π*(C^N)) and metal-to-ligand charge transfer (MLCT, dπ(Ir) → π*(C^N)) transitions. This is confirmed by theoretical calculations (Table 2 and Fig. 3). The absorption spectra of complexes Ir2–Ir4 incorporating the electron-donating diamine or phenolic group are red-shifted compared to Ir1. The presence of DPA moiety, which possesses the best electron-donating capability among the substituents incorporated in this work, makes the resulting complex Ir4 strongest absorber in the visible range. | ||
| Fig. 2 UV-vis absorption (left) and photoluminescence (right) spectra of complexes Ir1–Ir4 in DCM solutions at room temperature. | ||
| Complex | In DCM solutiona | In PMMA filmb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| λabs (nm) | λemc (nm) | Φd (%) | τd (μs) | λem (nm) | Φ (%) | τ (μs) | kre (×105 s−1) | knre (×105 s−1) | |
| a Measured in DCM solutions (1 × 10−5 mol L−1) at room temperature.b Measured in thin films doped in PMMA at 2% concentration.c Excitation wavelength = 400 nm.d The quantum yields (Φ) and lift time (τ) are recorded both in DCM solutions before (in bracket) and after deoxygenation.e kr and knr are calculated according to the equations: kr = Φ/τ and knr = (1 − Φ)/τ. | |||||||||
| Ir1 | 302, 430 | 602 | 15 (12) | 0.38 (0.17) | 596 | 26 | 1.53 | 1.70 | 4.84 |
| Ir2 | 253, 313, 361 | 586 | 26 (19) | 0.65 (0.21) | 584 | 73 | 2.28 | 3.20 | 1.18 |
| Ir3 | 311, 380, 450, 560 | 586 | 34 (20) | 0.79 (0.36) | 583 | 43 | 2.97 | 1.45 | 1.92 |
| Ir4 | 266, 318, 391 | 619 | 60 (40) | 0.70 (0.39) | 609 | 57 | 1.42 | 4.01 | 3.03 |
| Complex | Eoxa (V) | Ebandgapb (nm) | HOMOc (eV) | LUMOd (eV) |
|---|---|---|---|---|
| a Oxidation potentials recorded in deoxygenated DCM solutions with 0.1 M (nBu4NPF6) as supporting electrolyte, referenced to the Fc/Fc+ couple.b Ebandgap determined from the absorption edge of the iridium complexes.c HOMO levels calculated using the equation: HOMO = −[Eox − E(Fc/Fc+) + 4.8].d LUMO levels calculated using the equation: LUMO = HOMO + Ebandgap. | ||||
| Ir1 | 0.71 | 2.27 | −5.42 | −3.15 |
| Ir2 | 0.46 | 2.38 | −5.17 | −2.79 |
| Ir3 | 0.67 | 2.31 | −5.37 | −3.06 |
| Ir4 | 0.54 | 2.25 | −5.25 | −3.00 |
 | ||
| Fig. 3 Molecular orbital diagrams and energies for Ir1–Ir4 at their lowest singlet state (S0) (top) and first excited triplet state (T1) (bottom) geometries. | ||
All these complexes are photoluminescent in fluid DCM solutions at room temperature (Fig. 2). The complexes Ir1–Ir4 exhibit broad and structured-less emission originating from the mixed transitions of 3MLCT, 3LLCT and 3ILCT (intraligand charge transfer), which is demonstrated by the TD-DFT calculation shown below. The complex Ir1 shows an orange phosphorescent emission peak at 602 nm. Comparing with the complex Ir1, the emission maxima of Ir4 red-shifts about 17 nm, which owing to an increase of the π-conjugated system. Both Ir2 and Ir3 are blue-shifted with respect to Ir1 and exhibit intense yellow emission maxima at 586 nm. The almost overlap between the emission spectra of Ir2 and Ir3 indicates that both complexes emit from the lowest energy excited state which is not located on the substituted piperidyl or phenolic groups (see Theoretical studies section). The emission spectra of the complexes Ir1, Ir2 and Ir4 in PMMA doping films are similar to those in fluid DCM solutions, while Ir3 doping PMMA film exhibits structured emission spectrum at room temperature (Fig. S1†).
The phosphorescent lifetime and emission quantum yield of these complexes are investigated in both DCM solution and the thin film state (2 wt% in PMMA). The complex Ir1 exhibit a low quantum yield of 15% and 26% in deoxygenated DCM solutions and PMMA film, respectively. The incorporation of the diamine and phenolic substituents on the 2-thienyl quinazoline ligands provides steric hindrance and significantly increases the emission quantum yield of the complexes Ir2–Ir4 (Table 1). While the complexes Ir2 and Ir3 exhibit significant higher emission efficiency in PMMA doping film (Φ = 73% and 43%, respectively) than in deoxygenated solutions (Φ = 26% and 34%, respectively), the complex Ir4 shows similar emission quantum yield in both environments (Φ = 60% and 57%, respectively), indicating that the incorporation of DPA substituents in C^N ligands efficiently suppresses the aggregation of Ir4. The excited-state lifetimes of all these complexes are on the hundred-nanoseconds scale in DCM solutions and on the microseconds scale in solid PMMA doping films. It is well known that long lifetime will give rise to the emission saturation but usually causes the great triplet–triplet annihilation. Therefore, the suitable lifetime of these iridium complexes will be beneficial to improve the efficiency of OLEDs.
Electrochemical properties
The CV experiments are carried out using ferrocenium (Fc+)/Fc as the internal standard. All complexes exhibit reversible oxidation process in degassed DCM and show oxidation peaks ranging from 0.46 to 0.71 V (Fig. S2†). Based on the first oxidation potential and optical absorption edge of the UV-vis spectra, HOMO, LUMO and the HOMO–LUMO energy gaps (Eg) of Ir1–Ir4 are estimated (Table 2).11,14 The introduction of PD and DMP groups into the quinazolinyl ring results in larger increase of LUMO levels than HOMO levels due to the reduction process mainly involves the π* orbital of the quinazolinyl ring fragment while the oxidation process mainly involves the π orbital of the thienyl ring fragment. In the case of Ir4, the incorporation of DPA groups significantly extends the π-conjugated system of the C^N ligand, and promotes the HOMO level more than the LUMO level, leading to a slight decrease of Eg.Theoretical studies
To gain deeper insight into the photophysical and electrochemical properties of complexes Ir1–Ir4, DFT and TD-DFT calculations are carried out using the Gaussian 09 program package.15 Both the ground state (S0) and T1 optimized structures of Ir1–Ir4 are investigated. The calculated transition wavelength and main assignment are summarized in Table 3. The lowest energy absorption of Ir1 is mainly characterized by HOMO → LUMO transition. As shown in Fig. 3 and Table S4,† in the case of Ir1, the electron distribution of the HOMO is mainly located on both the whole C^N ligand (63.5%) and Ir(dπ) (33.0%), whereas that of LUMO primarily resides on one of the C^N ligands (91.7%). The lowest energy absorptions of Ir2–Ir4 are characterized by HOMO → LUMO and HOMO → LUMO+1 transitions. Their electron distributions of the HOMO are similar to each other, which are composed of the π orbital of the 2-thienyl quinazoline moiety of both the C^N ligand (62.2%, 62.8% and 64.2% for Ir2–Ir4, respectively) and Ir(dπ) (34.7%, 34.3% and 34.5% for Ir2–Ir4, respectively). All the substituents incorporated in the 2-thienyl quinazoline moiety have no electron distribution to the HOMO. It is observed that the LUMOs of Ir2–Ir4 are also predominately located on one of the C^N ligands (95.7%, 90.9% and 94.5% for Ir2–Ir4, respectively), which are similar to that of Ir1. The LUMO−1 of Ir3 and Ir4 is primarily contributed by the other C^N ligand (89.6% and 94.5% for Ir3 and Ir4, respectively), while that of Ir2 is composed of both the other C^N ligand and N^O ligand (97.1%). These results suggest that the lowest energy absorptions of Ir1–Ir4 can be ascribed to the mixture of π–π* and MLCT transition. The simulated absorption spectra (Fig. S3†) in DCM are almost consistent with the result of the experimental data.| Complex | State | λmax,cal (nm) | f | Main assignments | Character |
|---|---|---|---|---|---|
| Ir1 | S1 | 476.20 | 0.0294 | H → L (0.69520) | MLCT/LLCT |
| T1 | 713.63 | 0.0000 | H → L (0.59802); H−1 → L (0.25900); H → L+4 (−0.16374) | 3MLCT/3LLCT/3ILCT | |
| Ir2 | S1 | 444.47 | 0.0819 | H → L (0.67989); H → L+1 (−0.15104) | MLCT/LLCT |
| T1 | 689.60 | 0.0000 | H → L (0.61778); H−1 → L (0.25596); H → L+4 (0.10814) | 3MLCT/3LLCT/3ILCT | |
| Ir3 | S1 | 453.06 | 0.1029 | H → L (0.66627); H → L+1 (−0.20197) | MLCT/LLCT |
| T1 | 679.64 | 0.0000 | H → L (0.61449); H−1 → L (0.27359) | 3MLCT/3LLCT/3ILCT | |
| Ir4 | S1 | 475.87 | 0.0757 | H → L (0.68069); H → L+1 (0.16802) | MLCT/LLCT |
| T1 | 715.71 | 0.0000 | H → L (−0.59565); H−1 → L (−0.27210); H−3 → L (−0.17371) | 3MLCT/3LLCT/3ILCT |
The TD-DFT calculations at the T1 optimized geometry show that the lowest energy triplet state of Ir1–Ir4 is mainly composed of three transition configurations (two transition configurations for Ir3). The detailed information is listed in Table 3. As shown in Fig. 3 and Table S5,† the HOMO of Ir1–Ir4 is primarily located at the 2-thienyl quinazoline moieties of the both C^N ligands and iridium atom, which is similar to that in the ground state. As compared to the HOMO, the HOMO−1 of Ir1–Ir3 has more contribution from the both C^N ligands. However, the incorporation of DPA substituent on C^N ligand significantly affect the HOMO−1 of Ir4, which is mainly delocalized over one of the C^N ligand with the contribution form the DPA moiety (44.3%) and 2-thienyl quinazoline moiety (23.1%). The LUMOs of Ir1–Ir4 are predominately resides on one of C^N ligands (96.8%, 91.6%, 88.8% and 93.9%, respectively). The LUMO+4 of Ir1 and Ir2 exhibit similar electron distributions, primarily located on both the C^N ligands with more contributions from the quinazolinyl groups than thienyl groups. And the HOMO−3 of Ir4 is mainly contributed by both the C^N ligands with 42.3% contributions from the DPA moieties. These features indicate that the nature of T1 state of Ir1–Ir4 mainly consists of a mixture of 3MLCT, 3LLCT and 3ILCT (intraligand charge transfer). In addition, the vertical energy difference between T1 and S0 is computed by performing a single-point calculation of S0 at the optimized minimum-energy geometry of T1 to estimate the phosphorescence emission energy.16 The calculation results show that the vertical emission energies of these complexes are 1.84 eV (671.8 nm, Ir1), 1.89 eV (653.9 nm, Ir2), 1.90 eV (649.4 nm, Ir3) and 1.80 eV (687.3 nm, Ir4). Although these energies are underestimated compared to the experimental values, reasonable correlations can be found between the theoretical and experimental values for the maximum emission (Fig. S4†).
Conclusions
In summary, a strategy of electron structure and steric hindrance effect are incorporated to design and synthesise four new yellow to red phosphorescent iridium complexes containing TQZ derivatives. The introduction of appropriate electron-donating substituent (such as PD and DMP) to the 4 position of the quinazolinyl ring of TQZ ligand results in a different chelate manner, leading to significantly blue-shifts of phosphorescence band. Noted that the incorporation of bulky electron-donating substituents not only appropriately enhances the electron-donating effect without extending the conjugation of C^N ligands, but also provides steric hindrance around the iridium center to increase the PLQYs. Both of the electrochemical and theoretical studies suggest that a PD or DMP substituent on the quinazolinyl ring exhibits higher impact on the LUMO level than the HOMO level, resulting in a larger triplet excited state energy. All these results indicate that the 2-thienyl quinazoline based iridium complexes are potential high efficiency yellow/orange PhOLEDs materials. The strategy based on the synergistic effect of electron structure and hindrance offers an effective tool to adjust the opto-electronics properties in material design towards a bright future of organic electronics.Experiment section
Materials and methods
All commercial reagents were used without further purification, unless otherwise noted. N,N-dimethylacetamide (DMAc), and N,N-dimethylformamide (DMF) were dried by standard procedures before use. Dichloromethane (DCM) suitable for electrochemical and photospectral experiments was obtained by distillation over calcium hydride, silica gel (200–300 mesh, Qingdao Haiyang) was used for column chromatography.The 1H and 13C NMR (δ in ppm) spectra were recorded on a Bruker Ultra Shield Plus 400 MHz spectrometer with TMS as the internal standard and CDCl3 and DMSO-d6 as solvent. Mass spectra were acquired by a Bruker Daltonics matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF-MASS). UV-vis absorption spectra were recorded on a Shimadzu UV-3600 UV-vis-NIR Spectrophotometer. Steady state fluorescence spectra were obtained with a Shimadzu RF-5301PC Fluorescence Spectrophotometer. Photoluminescence quantum yields were determined by using an integrating sphere. Lifetime studies were performed with Edinburgh FL920 photocounting system with a semiconductor laser as the excitation source. For the thin film quantum yields and lifetime data measurements, materials (1 mg) were first dissolved in 1 mL of DCM containing PMMA (20 mg−1), and spin-coated at a speed of 1500 rpm for 30 s onto the quartz slides. Cyclic voltammetric (CV) measurements were carried out with a CHI660C electrochemical workstation (Shanghai Chenhua, China) utilizing the three electrode configuration consisting of a glassy carbon (working electrode), platinum wire (auxiliary electrode) and Ag/AgNO3 (reference electrode) electrodes. The experiments were done in dry DCM using 0.1 M tetrabutyl ammonium hexafluorophosphate as supporting electrolyte at a scanning rate of 0.1 V s−1 at room temperature. Ferrocene was selected as the standard. The crystallographic data for Ir1–Ir4 were collected on a EnrafNoius & EnrafNoius (CAD4/PC) at 296 K with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). The cell parameters were retrieved using Bruker SMART software and refined using Bruker SAINT on all observed reflections. All the calculations for the structure determination were carried out using the SHELXTL-97 crystallographic software package (Sheldrick, 1997) with a full-matrix least-squares technique based on F2.17 Hydrogen atoms were placed theoretically and rode on their parent atoms in the final refinement. Crystal data and processing parameters are summarized in Table S1, ESI.†
Computational details
The singlet ground-state (S0) geometries of Ir1–Ir4 were fully optimized by the Becke's three-parameter exchange functional and Lee–Yang–Parr correlation functional (B3LYP) method18 based on the experimental single crystal structural parameters. And the geometries of the lowest-lying triplet excited (T1) states were optimized by the unrestricted Becke's three-parameter hybrid exchange functional along with Lee–Yang–Parr correlation functional (UB3LYP) method.19 The electronic transition energies including electron correlation effects were computed by the time dependent density functional theory (TD-DFT) method using the B3LYP or UB3LYP functional.20 The LANL2DZ basis set was used for the iridium centers.21 For other atoms, 6–31g(d) basis sets were employed.22 TD-DFT approach was applied to predict their absorption and emission properties in DCM using the polarizable continuum model (PCM) approach.23Synthesis
Ir1. 61.7% yield; red solid. 1H NMR (400 MHz, DMSO) δ (ppm): 9.49 (s, 1H), 8.72 (s, 1H), 8.21 (d, J = 8.0 Hz, 1H), 8.15–8.04 (m, 2H), 8.02–7.85 (m, 5H), 7.77–7.64 (m, 3H), 7.67–7.50 (m, 3H), 6.45 (d, J = 4.8 Hz, 1H), 6.20 (d, J = 4.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 173.03, 167.99, 167.19, 159.68, 158.77, 150.77, 150.58, 149.09, 148.26, 138.42, 136.35, 136.30, 134.38, 132.82, 131.83, 131.11, 131.09, 128.68, 128.58, 128.11, 127.60, 127.38, 127.33, 126.74, 126.35, 120.98, 120.96. MS: (MALDI-TOF) m/z: 738.9 [M + 2H]+.
Ir2. 55.8% yield; red solid. 1H NMR (400 MHz, DMSO) δ (ppm): 8.26 (d, J = 8.0 Hz, 1H), 7.94 (m, 2H), 7.80 (dd, J = 12.1, 8.7 Hz, 3H), 7.71 (m, 1H), 7.53 (dd, J = 11.5, 4.2 Hz, 1H), 7.47 (s, 1H), 7.36 (t, J = 7.2 Hz, 1H), 7.27 (s, 2H), 7.14 (t, J = 7.3 Hz, 1H), 6.75 (d, J = 8.3 Hz, 1H), 6.23 (s, 1H), 5.90 (s, 1H), 4.04–3.75 (m, 8H), 1.96–1.56 (m, 12H). 13C NMR (100 MHz, CDCl3) δ (ppm): 172.76, 169.84, 169.34, 164.54, 164.37, 153.07, 152.28, 151.82, 150.91, 147.56, 139.09, 137.17, 136.67, 134.14, 134.10, 132.75, 132.24, 130.79, 129.62, 127.69, 127.21, 126.03, 125.45, 124.95, 123.62, 122.55, 122.42, 113.28, 112.86, 50.79, 50.61, 31.58, 26.19, 26.15, 24.79, 24.71, 22.65, 14.12. MS: (MALDI-TOF) m/z: 905.5 [M + 2H]+.
Ir3. 31.4% yield; red solid. 1H NMR (400 MHz, DMSO) δ (ppm): 8.35 (m, 3H), 7.98 (dd, J = 7.6, 1.5 Hz, 1H), 7.86–7.75 (m, 3H), 7.75–7.67 (m, 1H), 7.63–7.48 (m, 3H), 7.41–7.29 (m, 2H), 7.29–7.17 (m, 6H), 6.71 (d, J = 8.8 Hz, 1H), 6.47 (d, J = 4.8 Hz, 1H), 6.14 (d, J = 4.7 Hz, 1H), 2.11 (d, J = 60.0 Hz, 12H). 13C NMR (100 MHz, CDCl3) δ (ppm): 172.53, 171.91, 170.96, 166.65, 153.08, 153.07, 152.95, 152.53, 152.10, 149.62, 149.60, 147.10, 139.33, 137.95, 136.82, 136.29, 134.46, 133.73, 133.25, 132.59, 132.33, 127.93, 127.63, 126.03, 126.01, 125.88, 125.07, 124.89, 124.44, 123.98, 121.80, 113.02, 112.34, 16.77. MS: (MALDI-TOF) m/z: 979.8 [M + 2H]+.
Ir4. 59.7% yield, red solid. 1H NMR (400 MHz, DMSO) δ (ppm): 8.33 (d, J = 8.6 Hz, 1H), 8.01 (m, 1H), 7.88 (d, J = 4.8 Hz, 1H), 7.83 (dd, J = 4.5, 4.0 Hz, 1H), 7.79–7.66 (m, 1H), 7.53–7.38 (m, 10H), 7.36–7.14 (m, 15H), 7.12–6.92 (m, 3H), 6.76–6.67 (m, 1H), 6.40 (d, J = 4.8 Hz, 1H), 6.07 (d, J = 4.7 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 172.68, 172.67, 170.73, 170.02, 163.44, 163.37, 153.13, 152.42, 151.32, 147.56, 146.40, 146.38, 139.35, 137.55, 136.78, 134.58, 133.87, 132.64, 132.54, 131.95, 130.90, 129.48, 129.45, 127.01, 126.68, 126.16, 126.10, 114.53, 113.97. MS: (MALDI-TOF) m/z: 1073.9 [M + 2H]+.
Acknowledgements
The authors acknowledge financial support from the National Basic Research Program of China (973 Program, 2012CB933301, 2012CB723402), the Ministry of Education of China(IRT1148), the National Natural Science Foundation of China (BZ2010043, 21572001, 20974046, 20774043, 51173081, 50428303, 61136003), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, YX03001), Synergetic Innovation Center for Organic Electronics and Information Displays, Natural Science Foundation of Jiangsu Province, China (BM2012010), the Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP) (20113223110005), Jiangsu Planned Projects for Postdoctoral Research Funds(1402010B) and the Research Fund for Nanjing University of Posts and Telecommunications (NY213177).Notes and references
- W. Y. Wong and C. L. Ho, Coordin. Chem. Rev., 2009, 253, 1709 CrossRef CAS; Y. Chi and P. T. Chou, Chem. Soc. Rev., 2010, 39, 638 RSC; L. Xiao, Z. Chen, B. Qu, J. Luo, S. Kong, Q. Gong and J. Kido, Adv. Mater., 2011, 23, 926 CrossRef PubMed.
- C. Fan and C. L. Yang, Chem. Soc. Rev., 2014, 43, 6439 RSC.
- H. H. Chou and C. H. Cheng, Adv. Mater., 2010, 22, 2468 CrossRef CAS PubMed; Y. J. Cho and J. Y. Lee, Adv. Mater., 2011, 23, 4568 CrossRef PubMed; C. H. Fan, P. Sun, T. H. Su and C. H. Cheng, Adv. Mater., 2011, 23, 2981 CrossRef PubMed.
- J. Zou, H. Wu, C. S. Lam, C. Wang, J. Zhu, C. Zhong, S. Hu, C. L. Ho, G. J. Zhou, H. Wu, W. C. Choy, J. Peng, Y. Cao and W. Y. Wong, Adv. Mater., 2011, 23, 2976 CrossRef CAS PubMed.
- K. Dedeian, J. Shi, N. Shepherd, E. Forsythe and D. C. Morton, Inorg. Chem., 2005, 44, 4445 CrossRef CAS PubMed; G. Zhou, C.-L. Ho, W.-Y. Wong, Q. Wang, D. Ma, L. Wang, Z. Lin, T. B. Marder and A. Beeby, Adv. Funct. Mater., 2008, 18, 499 CrossRef; M. Zhu, Y. Li, S. Hu, C. Li, C. Yang, H. Wu, J. Qin and Y. Cao, Chem. Commun., 2012, 48, 2695 RSC; Q. L. Xu, C. C. Wang, T. Y. Li, M. Y. Teng, S. Zhang, Y. M. Jing, X. Yang, W. N. Li, C. Lin, Y. X. Zheng, J. L. Zuo and X. Z. You, Inorg. Chem., 2013, 52, 4916 CrossRef PubMed.
- F. M. Hwang, H. Y. Chen, P. S. Chen, C. S. Liu, Y. Chi, C. F. Shu, F. I. Wu, P. T. Chou, S. M. Peng and G. H. Lee, Inorg. Chem., 2005, 44, 1344 CrossRef CAS PubMed; P. J. Hay, J. Phys. Chem. A, 2002, 106, 1634 CrossRef; S. Lamansky, P. Djurovich, D. Murphy, F. Abdel-Razzaq, H.-E. Lee, C. Adachi, P. E. Burrows, S. R. Forrest and M. E. Thompson, J. Am. Chem. Soc., 2001, 123, 4304 CrossRef PubMed; V. V. Grushin, N. Herron, D. D. LeCloux, W. J. Marshall, V. A. Petrov and Y. Wang, Chem. Commun., 2001, 1494 RSC; P. I. Djurovich, D. Murphy, M. E. Thompson, B. Hernandez, R. Gao, P. L. Hunt and M. Selke, Dalton Trans., 2007, 3763 RSC.
- T. Liu, B.-H. Xia, X. Zhou, Q.-C. Zheng, Q.-J. Pan and H.-X. Zhang, Theor. Chem. Acc., 2008, 121, 155 CrossRef CAS; T. Kim, H. Kim, K. M. Lee, Y. S. Lee and M. H. Lee, Inorg. Chem., 2013, 52, 160 CrossRef PubMed.
- L. H. Xie, C. R. Yin, W. Y. Lai, Q. L. Fan and W. Huang, Prog. Polym. Sci., 2012, 37, 1192 CrossRef CAS.
- W. L. Yu, J. Pei, W. Huang and A. J. Heeger, Adv. Mater., 2000, 12, 828 CrossRef CAS.
- L. H. Xie, R. Zhu, Y. Qian, R. R. Liu, S. F. Chen, J. Lin and W. Huang, J. Phys. Chem. Lett., 2010, 1, 272 CrossRef CAS.
- Q. Mei, L. Wang, Y. Guo, J. Weng, F. Yan, B. Tian and B. Tong, J. Mater. Chem., 2012, 22, 6878 RSC.
- Y. Fang, S. J. Hu, Y. Z. Meng, J. B. Peng and B. Wang, Inorg. Chim. Acta, 2009, 362, 4985 CrossRef CAS; J. S. Parka, M. K. Songa, Y. S. Gal, J. W. Lee and S. H. Jin, Synth. Met., 2011, 161, 213 CrossRef.
- J. S. Parka, M. K. Songa, Y. S. Gal, J. W. Lee and S. H. Jin, Synth. Met., 2011, 161, 213 CrossRef.
- X. Zhang, J. Gao, C. Yang, L. Zhu, Z. Li, K. Zhang, J. Qin, H. You and D. Ma, J. Organomet. Chem., 2006, 691, 4312 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- F. Kessler, R. D. Costa, D. D. Censo, R. Scopelliti, E. Ortí, H. J. Bolink, S. Meier, W. Sarfert, M. Grtäzel, M. K. Nazeeruddin and E. Baranoff, Dalton Trans., 2012, 41, 180 RSC.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2008, 64, 112 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1988, 88, 2547 CrossRef CAS; A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 270 CrossRef CAS; P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299 CrossRef.
- E. Runge and E. K. U. Gross, Phys. Rev. Lett., 1984, 52, 997 CrossRef CAS.
- S. J. Liu, N. N. Song, J. X. Wang, Y. Q. Huang, Q. Zhao, X. M. Liu, S. Sun and W. Huang, Phys. Chem. Chem. Phys., 2011, 13, 18497 RSC.
- W. J. Hehre, J. Chem. Phys., 1972, 56, 2257 CrossRef CAS; P. C. Hariharan and J. A. Pople, Theoret. Chim. Acta, 1973, 28, 213 CrossRef; M. M. Francl, J. Chem. Phys., 1982, 77, 3654 CrossRef.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1402154–1402157. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra17437f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |