Synthesis and characterization of blue TiO2/CoAl2O4 complex pigments with good colour and enhanced near-infrared reflectance properties
Wei Zheng and
Jian Zou*
School of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, PR China. E-mail: ezouj@swu.edu.cn
First published on 12th October 2015
Abstract
Blue TiO2/CoAl2O4 complex pigments were prepared through calcination of precursors from the precipitation of Al3+ and Co2+ on TiO2 particles in sequence. The synthesized powders were characterized by colorimetry, near-infrared diffuse reflectance spectroscopy, X-ray diffraction, scanning electron microscopy and ultraviolet-visible spectroscopy. The pigments were found to have composite phases composed of rutile TiO2 and spinel CoAl2O4. The bright blue pigments having good color properties could be obtained via calcination of the precursors at 1000 °C. As the mass of CoAl2O4 increased to 40 wt% of TiO2, the pigments presented good color properties (L* = 53.43, a* = −4.75, b* = −41.78) and the results showed little variation with an increase in the CoAl2O4 content. In comparison to the pure CoAl2O4 pigments, the as-prepared pigments with a CoAl2O4/TiO2 mass ratio of 0.4 exhibited an enhanced near-infrared reflectance and also showed better color properties relative to the mixed pigments of TiO2 and CoAl2O4.
Introduction
Cobalt aluminate (CoAl2O4), which is commonly known as Thenard's blue, has an impressive optical effect. It is characterized by a spinel-type structure and has unique properties. Some of these unique properties are high refractive index, chemical reactivity, colour and good thermal stability.1 Cobalt aluminate is widely used in the production of ceramics, plastics, paint, rubber and glass. Various processes have been developed for the synthesis of cobalt blue. Some of these processes are solid-phase reaction,2 sol–gel,2–7 co-precipitation,8 sonochemical synthesis,9 hydrothermal synthesis,4,10 complexation11 and combustion.1 With the recent development of material synthesis technology, nano-sized cobalt blue pigments can also be prepared via a number of special methods.12–14 The pigments resulting from these methods can be transparent13 and highly stable in different media.15 These new methods can help find new applications for the cobalt blue. One example of such development and application is of using cobalt blue in the production of the ceramic ink-jet printing ink.12In order to enhance the aesthetics of the built environment, dark-coloured pigments are often combined with the surface coatings for their applications to the buildings as paints.16,17 Most of the solar energy (52% of the irradiance) lies in the near – infrared region (700–2500 nm).16,17 Since most of the dark pigments have low near – infrared (NIR) reflectance, therefore the surface temperature of the built environment increases under solar irradiation due to the NIR absorption. The increase in temperature decreases the comfort in the inner rooms and hence results in an increase in the energy consumption due to a higher use of air conditioning. On the other hand, preferring cool pigments over the traditional pigments, results in a dramatically lower heat build-up in the surface of the roofs.18–20 In one of our previous works,21 it was demonstrated that the application of the cool NIR reflection pigments can reduce the surface temperature by about 10 °C. In addition, under low wind conditions, the average air temperature on a road can be reduced by 5 °C by replacing the conventional asphalt with cool pigments.22 Thus, various synthesis methods have been adopted to enhance the NIR reflectance of pigments or to develop novel colour pigments having similar characteristics. One of the methods is to dope the pigments with different metal ions in order to enhance their NIR reflectance.23–27 The NIR reflectance of Cr2O3 green pigments can be improved from 55% to 85% by doping it with lanthanum (La) and praseodymium (Pr) ions.23 The NIR reflectance of BiVO4 yellow pigments can also be improved from 50% to 88% by applying the doping technology.24–26 In addition, due to low toxicity28,29 and high NIR reflectance, several rare metal-based pigments have been proposed as viable alternatives to the traditional toxic pigments. The NIR reflectance of such pigments can exceed the value of 90%. Y2BaCuO5 (ref. 30) and BaCr2(P2O7)2 (ref. 31) were developed as green pigments having 90% NIR reflectance. Moreover, SrCuSi4O10 doped with Fe, La and Li not only exhibit high NIR reflectance, but also show better colour properties relative to the cobalt blue.32
Due to its high reflectance in the short NIR region, cobalt aluminate can also be used as a cool pigment.33 However, cobalt is scarce and expensive. Furthermore, serious environmental problems may arise from the manufacture of Co-based ceramic pigments.34 Cobalt blue also exhibits an undesirable absorption band in the 1200–1600 nm range.33 Hence, NIR reflectance of the cobalt blue must be improved while the use of cobalt in the development of the cool cobalt blue pigment should be reduced. Rutile TiO2 is a white pigment having a high NIR reflectance. When it is used in mixed pigments, it has the ability to further improve the NIR reflectance.35,36 Rutile TiO2 also decreases the use of CoAl2O4 which is used in both the mixed pigments and in colour enhancing schemes for the pigments, but the mixture with high NIR reflectance tend to be light in colour due to the diluting effect of TiO2.35–37 The doping technology can enhance the NIR reflectance of pigments,24–26 but the colour hues of CoAl2O4 probably are changed.38 A two-layer process has been proposed as an alternative to the mixed pigment coatings.17,37 The coatings resulting from the two-layer process exhibit high NIR and have the same visible aspects as those of the common building surfaces. In the present study, a complex pigment consisting of TiO2 core and CoAl2O4 outer layer was also prepared for the cool products. Complex pigments with low cobalt consumption are expected to exhibit not only improved NIR reflectance but also good colour properties.
Experimental
Materials
CoCl2·6H2O, Al(NO3)3·9H2O and NH3·H2O (25%–28%) were purchased from the Chuandong Chemical Co., Ltd., China. All reagents were analytically pure. Other reagents, such as polyethylene glycol (PEG 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), titanium tetrachloride (TiCl4, Sinopharm Chemical Reagent Co. Ltd., China) and polyurethane (PU) paint (Dü Fang, Acryl PU-Klalack) were used as received.
000), titanium tetrachloride (TiCl4, Sinopharm Chemical Reagent Co. Ltd., China) and polyurethane (PU) paint (Dü Fang, Acryl PU-Klalack) were used as received.
Synthesis of complex pigments
TiO2 powder was synthesized according to a process similar to the one reported in the ref. 21 and 39. In a typical synthesis, 20 mL of TiCl4 was added to the ice blocks along with vigorous stirring. Once the ice blocks melted completely, the solution was diluted to a volume of 178 mL. Subsequently, 1 wt% of PEG 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, which acted as a dispersant, was added to the solution. After refluxing the solution at 100 °C for 4 h, the pH of the suspension was adjusted to about 6 by using 10% aqueous ammonia. The TiO2 powder was obtained by filtering and washing the suspension several times followed by a drying process at 90 °C for 3 h.
000, which acted as a dispersant, was added to the solution. After refluxing the solution at 100 °C for 4 h, the pH of the suspension was adjusted to about 6 by using 10% aqueous ammonia. The TiO2 powder was obtained by filtering and washing the suspension several times followed by a drying process at 90 °C for 3 h.
About 2 g of the as-prepared TiO2 powder was re-dispersed in 50 mL of the distilled water under vigorous stirring. Aluminum nitrate was dissolved in this suspension and a 10% aqueous ammonia solution was used to adjust the pH to a value which lied in the range of 6–7. Subsequently, 1 M cobalt chloride was added to the suspension drop by drop and the pH of the suspension was adjusted to a value of around 9–10 by adding 10% aqueous ammonia solution. The precipitate was obtained after several cycles of filtering and washing processes. The resulting powder was obtained by drying the precipitate at 90 °C for 4 h. The colour products were obtained by calcination of the powder at different temperatures ranging from 700 °C to 1000 °C. For all pigments, the ratio of Al3+ to Co2+ was kept constant at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This value has been reported as the optimum value for the Al3+ to Co2+ ratio.2 The contents of cobalt chloride were determined by the contents of theoretical CoAl2O4 which was used in mass ratios of 0.2, 0.4, 0.6, 0.8 and 1.0 relative to TiO2. Pure CoAl2O4 pigments were obtained by calcining the precipitates of Al3+ and Co2+ without TiO2 powder at 1000 °C. The mixed pigments of CoAl2O4 and TiO2 were acquired by direct mixing of CoAl2O4 and TiO2 powder. The CoAl2O4 content was measured from the CoAl2O4/TiO2 ratio which had a value of 0.4.
1. This value has been reported as the optimum value for the Al3+ to Co2+ ratio.2 The contents of cobalt chloride were determined by the contents of theoretical CoAl2O4 which was used in mass ratios of 0.2, 0.4, 0.6, 0.8 and 1.0 relative to TiO2. Pure CoAl2O4 pigments were obtained by calcining the precipitates of Al3+ and Co2+ without TiO2 powder at 1000 °C. The mixed pigments of CoAl2O4 and TiO2 were acquired by direct mixing of CoAl2O4 and TiO2 powder. The CoAl2O4 content was measured from the CoAl2O4/TiO2 ratio which had a value of 0.4.
Coloration of plastics and paints
Approximately 0.2 g of the pigments was mixed with 10 g of PU paint under ultrasonic treatment for 2 min. The mixture was then placed in a 9 cm Petri dish and allowed to solidify. Coloured paint was obtained after solidification.Characterization techniques
The phases of the products were characterized by X-ray diffraction (XRD) using Cu-Kα radiation in an XD-3 diffractometer (Beijing Pgeneral). Morphological analyses were performed by scanning electron microscopy (Quanta x50 FEG). Ultraviolet-visible (UV-vis) NIR spectra were recorded on a spectrophotometer with an integrated sphere (Hatachi U-4100). For UV-vis analysis, BaSO4 was used as the reference sample. The colour of the pigments was evaluated by measuring the L*, a* and b* parameters by using a Konica-Minolta spectrophotometer CM-700d. Near-infrared diffuse reflectance spectroscopy was conducted to confirm the optical properties of the samples. The micrographs were obtained using an Olympus CX31 instrument. Thermogravimetry and differential scanning calorimetry (TG-DSC) of the pigments were carried out in air with a heating rate of 10 °C min−1 by using Netzsch instruments (NETZSCH STA 409 PC/PG).Results and discussion
The phase composition and morphology of the as-prepared pigments
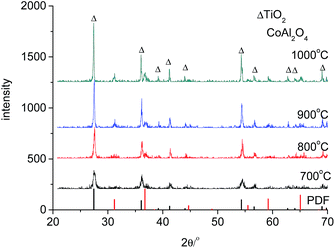
The obtained TiO2/CoAl2O4 complex pigments were expected to show blue colour and an enhanced NIR reflectance. Therefore, the expected phase composition for these pigments was rutile titania and spinel cobalt aluminate. As shown in Fig. 1, the rutile TiO2 phase with the representative peaks at around 27.4, 36.1 and 54.3° was observed for all products, which were calcined in the range of 700–1000 °C. As the calcination temperature was increased, the peaks became more intense and sharper, indicating improved crystallinity. The calcination temperature is also a key factor in the formation of spinel cobalt aluminate. During its formation, a higher temperature resulted in the formation of more cobalt aluminate. Fig. 1 shows that the spinel CoAl2O4 phase with the representative peaks at around 31.2 and 36.8° was distinctive only above 900 °C. The most intensive peak occurred for the sample which was calcined at 1000 °C. However, the products, which were calcined at 700 °C and 800 °C, did not show any spinel cobalt aluminate phase. No peaks corresponding to the cobalt oxides and alumina were observed. This indicated that the resulting oxides might be amorphous in nature. Similar results have also been reported in a previous study.40 The cobalt aluminate phase can be obtained by heating the samples above the formation temperature of the blue CoAl2O4. Zayat et al.2 observed that the formation of the CoAl2O4 phase occurred only when the temperature was above 800 °C. Alternatively, Cava et al.40 found that the cobalt aluminate phase was formed at a temperature above 1050 °C.The existence of spinel CoAl2O4 determined the coloration of the complex pigments. The phase compositions of the pigments with different CoAl2O4 contents are shown in Fig. 2. The sample having a CoAl2O4/TiO2 mass ratio of 0.2 showed a significant spinel CoAl2O4 phase. An increase in the CoAl2O4 content resulted in more intensive peaks for the CoAl2O4 phase. These results indicated an increase in the CoAl2O4 phase in the pigments. On the other hand, the peaks for the rutile TiO2 phase decreased. In addition, no phases other than the spinel CoAl2O4 and rutile TiO2 phases were detected. Typically, precipitates of Co2+ would react with Al3+ precipitates to form CoAl2O4 above the formation temperature of CoAl2O4.2 However, the same reaction can occur between the precipitates of Co2+ and TiO2 to form cobalt titanate. Two of such examples include CoTiO3 (ref. 41 and 42) and Co2TiO4.41 Sales et al.43 observed the formation of the CoTiO3 phase for gels with Co, Al and Ti. Cobalt titanate exhibited a dark green colour,16,42 which deteriorated the colour properties of the pigments. However, no cobalt titanate was detected in all of the samples (as shown in the XRD patterns presented in Fig. 2). These results indicate the isolation of a rich aluminum coating from the TiO2 and the precipitates of Co2+. Therefore, the expected phase composition of the obtained pigments showed good colour properties and high values of reflectance.
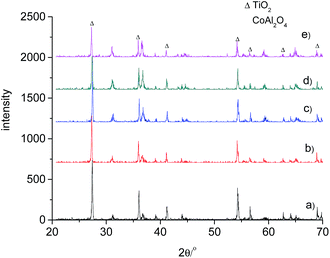 | ||
| Fig. 2 XRD patterns of the samples with different mass ratios of CoAl2O4/TiO2 at 1000 °C. (a) 0.2, (b) 0.4, (c) 0.6, (d) 0.8, (e) 1. | ||
The dispersion and grain size of pigment particles can be detected by SEM. Fig. 3 shows SEM image of the complex pigment with 0.4 mass ratio of CoAl2O4/TiO2. The pigment particles were granular in nature and presented a homogeneous grain size. The grain sizes were distributed from 1 μm to 5 μm. Compared with the pigments in precious report,21 these pigment particles have bigger particle size and present irregular morphology. And some smaller particles anchored on the surface of these irregular particles. All these differences of morphology and grain size plausibly come from the formation of spinel Co2AlO4.
The optical and colour properties of pigments
Different phase compositions may result in different absorptions in the visible region. The pigments showed different hues. Optical properties of the as-prepared complex pigments were studied by measuring the UV-vis spectra. Results from the analysis of UV-vis spectra of various samples, which were calcined at different temperature, showed that with an increase in the calcination temperature, the CoAl2O4 phase became more evident (as shown in Fig. 4a). The energy level for Co2+ (3d7 configuration) in both octahedral and tetrahedral ligand fields has three spin-allowed transitions.2,13 The spectra showed a band absorption pattern at around 546, 584 and 624 nm. The triple band was attributed to a Jahn–Teller distortion of the tetrahedral structure,13,40,44 which is responsible for the blue coloration. The spin-forbidden transition was observed as small peaks or shoulders around 479 nm and was attributed to transitions between the octahedral and tetrahedral sites.44 The observed absorption bands became more intense as the calcination temperature increased, thereby indicating that the CoAl2O4 phase increased with an increase in the temperature. This behaviour was consistent with the XRD results. Certainly, the above finding hinted that the blue hue value of the pigments would increase with the calcination temperature.In addition, the absorption bands between 400–500 nm and above 700 nm were also analysed. These absorption bands corresponded to the samples which were calcined at lower temperatures, especially for the sample calcined at 700 °C. The absorption bands were due to the Co3+ component of Co3O4,45,46 which would result in a dark green colour of the obtained pigments 2. Indeed, the sample, which was calcined at 700 °C, showed a grey green colour. The bands decreased and disappeared with an increase in the calcination temperature. Simultaneously, the observed colour of the pigments changed from green to bright blue. These findings suggested that the preparation temperature determined the coloration of the pigments. This was due to the CoAl2O4 and Co3O4, both of which depend upon the calcination temperature.
To obtain the CoAl2O4 phase, pigments must undergo calcination at 1000 °C. The optical spectra of the pigments with different CoAl2O4 contents are presented in Fig. 4b. Fig. 4b showed that all samples exhibited the characteristic absorption band of CoAl2O4 phase only, indicating that the pigments exhibited the expected bright blue colour. The absorption band at 350–400 nm was attributed to the absorption of TiO2 in complex pigments, which decreased with an increase in the CoAl2O4 contents. This band was located in the ultraviolet region, so the difference did not result into a different colour. In general, the visible absorption of the complex pigments depends on the CoAl2O4 phase content, in which the pigments having lesser CoAl2O4 content show lower absorption. Indeed, the pigment with a CoAl2O4/TiO2 mass ratio of 0.2 showed much lower visible absorption compared to other pigments. These results suggested that this pigment demonstrated the lowest intensity of colours. Interestingly, when the CoAl2O4 content increased above 0.4, the differences in absorption intensity were hardly distinguishable. Thus, the visible absorption intensity of pigments was not always as heavily dependent on the CoAl2O4 content as expected.
The colour properties of the pigments were investigated further by colorimetry. Fig. 5 shows the colorimetric parameters of the obtained pigments. As shown in Fig. 1 and 4a, the calcination temperature determined both the phase composition and the visible absorption of the obtained pigments. Therefore, the calcination temperature played a critical role in the colour properties of the pigments. Fig. 5a shows that the luminosity L* increased slightly with an increase in the calcination temperature. All samples exhibited green (−a*) and blue (−b*) hues. The green component (−a*) decreased as the temperature increased, whereas an opposite trend (between the hue and the temperature) was observed for the blue component (−b*). As shown in Fig. 5a, the sample which was calcined at 700 °C presented a nearly pure dark green colour (L* = 46.46, b* = −1.75, a* = −15.15) while the sample, which was calcined at 1000 °C, had a bright blue colour and showed the highest luminosity (L* = 53.43), blue component (b* = −41.78) and the lowest green component (a* = −4.75). High calcination temperature enhanced the occurrence of the blue components and the elimination of green hue. This could be attributed to the formation of CoAl2O4 and the exhaustion of Co3O4 respectively. These findings were consistent with the results of both the XRD and the optical absorption.
 | ||
| Fig. 5 Colourimetric parameters of pigments with 0.4 mass ratios of CoAl2O4/TiO2 calcined at different temperatures (a) and with different mass ratios of CoAl2O4/TiO2 by calcination of 1000 °C (b). | ||
The CoAl2O4 phase is responsible for the blue hue of the pigments. The results in XRD and optical absorption indicated that the CoAl2O4 phase occurred for all pigments which were calcined at 1000 °C. Therefore, as shown in Fig. 5b, pigments with good colour properties could be obtained with as low as 20 wt% CoAl2O4 relative to TiO2. As the CoAl2O4 content increased, both the luminosity L* and the green component (−a*) decreased while the blue component (−b*) showed an increasing trend. However, compared to the calcination temperature, the effect of the CoAl2O4 content on the colour properties was considerably less significant, especially when the CoAl2O4 content increased above 0.4. This finding was consistent with the results of the optical absorption presented in Fig. 4b. For blue CoAl2O4 pigments, Zayat et al.2 observed that the formation of white Al2O3 from excess Al in the pigments results in a loss of colour intensity. In comparison to the pure CoAl2O4 pigments (L* = 30, a* = 9.52, b* = −48.61), the mixed pigments with a CoAl2O4/TiO2 mass ratio of 0.4 presented much lower colour intensity (L* = 65.97, a* = −2.31, b* = −28.11). This result was attributed to the dilution effect of white TiO2 in the mixed pigments.35,36 Similarly, in comparison to the pure blue pigment, the complex pigments with a mass ratio of 0.4 presented slightly lower colour intensity (b* = −41.78). However, even though the complex pigments and the mixed pigments contained equal contents of CoAl2O4, yet the intensity of the complex pigments was much higher than that of the mixed pigments. In comparison to the pure pigment, the complex pigments showed much higher luminosity L*. More importantly, the complex pigments with good properties (L* = 53.43, a* = −4.75, b* = −41.78) effectively contained 28.5% CoAl2O4. This meant that the mass ratio of CoAl2O4/TiO2 was 0.4. Therefore, the complex pigments with good properties could reduce the use of Co.
As shown in Fig. 5, the obtained complex pigments exhibited good properties. The vivid bright blue colour of the complex pigment with a CoAl2O4/TiO2 mass ratio of 0.4 is shown in Fig. 6. However, the mixed pigment with the same CoAl2O4 content showed a light blue colour. This visual difference was consistent with the results of the colorimetric parameters. The complex pigments showed high tinting strength. A film of PU paint coloured with the complex pigments exhibited a similar colour. No further processing was implemented for the complex pigment. The coloured PU film exhibited a homogeneous colour, indicating that the complex pigment was homogeneously dispersed in the organic matrix.
 | ||
| Fig. 6 Photos of complex pigment (a), PU colored with the complex pigment (b) and the mixed pigments with the same contents of CoAl2O4 (c). | ||
The thermal stability and chemical resistance of the pigments
The thermal behaviour of the complex pigment was studied for a temperature change ranging from room temperature to 900 °C (as shown in Fig. 7a). The TG curve illustrated a slight loss of 2.3%, which could be attributed to the release of physically adsorbed water. No endothermic peak was observed from the DSC curve, indicating that no phase transition occurred for the complex pigment. We also analysed the chemical stability of the complex pigment via comparative acid-corrosion experiment in 1 M HCl for 96 h. Fig. 7b shows the absorbance curves of 0.01 M CoCl2 and 1 M HCl solution with complex pigment. The CoCl2 solution presented absorption at 511 nm, but no absorption was observed for HCl solution with the complex pigment. This indicated that the complex pigment had high chemical resistance to HCl solution. Obviously, the good thermal behaviour and high chemical resistance of the complex pigments are beneficial for their potential applications.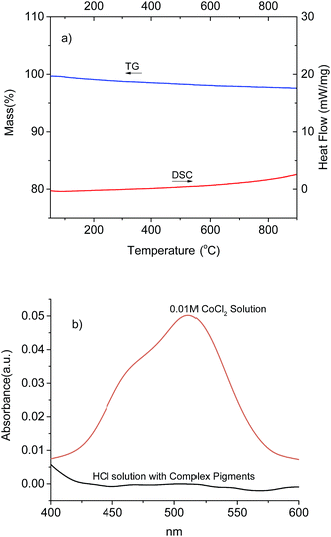 | ||
| Fig. 7 TG-DSC curves of the complex pigment with 0.4 mass ratios of CoAl2O4/TiO2 (a) and the absorbance curves of CoCl2 solution and HCl solution with complex pigment (b). | ||
The NIR reflectance of the pigments
Around 52% of the energy in the solar irradiance spectrum is from near infrared irradiation (700–2500 nm).16,17 For the cool nonwhite pigments, this part of solar energy is expected to be reflected as much as possible. Doping is often used to enhance the NIR reflectance of nonwhite pigments.23–27 Another method to enhance the NIR reflectance is to colour the white pigments with high NIR reflectance by the coloured pigments and hence, prepare mixed pigments.35,36 As shown in Fig. 8, a visible improvement in the NIR reflectance was observed for the mixed pigments, which could be attributed to the high reflection of rutile TiO2. However, the Fig. 6 and the results of the colorimetric parameters show that this enhancement was at the expense of colour intensity, which can be confirmed by the results presented in the Fig. 8. The mixed pigment had the highest reflectance in the range of 500–700 nm. Other published reports have also presented similar findings.35,36 Therefore, the deep colour coating could not be obtained by using the mixed pigments, but the advantage lies in acceptable aesthetics of the dark colours.47 The above results showed that the complex pigments exhibited good properties and enhanced reflectance (as shown in Fig. 8). In comparison to the pure CoAl2O4, the complex pigment showed at least 10% enhancement in the NIR reflectance. At the characteristic absorption band (1200–1600 nm) of CoAl2O4,33 the complex pigment showed around 15% enhancement and ultimately increased to 30% in the range of 1700–2500 nm. Furthermore, the reflectance of the complex pigment was observed to be 10% lower than that of the mixed pigment. However, the energy of the observed band accounted for 17% of the solar energy,33 which demonstrated that the solar reflectance of the complex pigment was only 1.7% lower than that of one of the mixed pigments. Therefore, the complex pigments exhibited good colour properties and high NIR reflectance.The above results indicated that the complex pigments exhibited a higher NIR reflectance and better colour properties relative to the pure cobalt blue pigments and mixed pigments with the same compositions. In this work, the CoAl2O4 phase was precipitated on the surface of rutile TiO2. Therefore, the complex pigment consisted of CoAl2O4 on the surface and rutile TiO2 in the inner core. The CoAl2O4 phase on the surface resulted in the blue hue of the complex pigment particles. As shown in Fig. 9a, the micrograph of the complex pigment exhibited an almost uniform blue hue. However, for the mixed pigments with the same compositions, a completely different micrograph was observed (see Fig. 9b). The mixed pigments consisted of the blue CoAl2O4 aggregates and yellow rutile TiO2. The vast majority of the rutile TiO2 could shield part of the CoAl2O4 to absorb some visible light and NIR. Therefore, a lighter blue hue and a higher NIR (1200–1600 nm) reflectance were observed for the mixed pigments (as shown in Fig. 6c and 8 respectively). Some similar conclusions have been reported in previous studies.35,36 Jiang et al.35 mixed white rutile TiO2 and cobalt aluminate blue to obtain a colourful cool coating having high solar reflectance. However, the coating Jiang et al.35 prepared was light blue grey in colour. A grey coating with high solar reflectance could be obtained by mixing black pigments and TiO2.36
 | ||
| Fig. 9 Micrograph of the samples. (a) Complex pigments with 0.4 mass ratios of CoAl2O4/TiO2 and (b) mixed pigment with the same content of CoAl2O4. The bar represents 20 μm. | ||
A two-layer coating process has recently been used to prepare both colourful and darker coatings with high NIR reflectance.17,37 Libbra et al.17 coated the colour coating on the NIR-reflective basecoat to prepare cool colour red clay tile having higher solar reflectance. The cool colour pigments prepared by Libbra et al.17 yielded similar visible aspects as those of common building surfaces. Levinson et al.37 also generated cool coloured concrete tiles and asphalt shingle roofing products having a dark appearance. For the complex cobalt aluminum blue pigments, a similar two-layer structure was obtained, which meant that the outer CoAl2O4 would render pigments with a bright blue colour. The outer CoAl2O4 would also render inner rutile TiO2 core, which was one of the pigments, with over 80% NIR reflectance,21 hence enhancing the NIR reflectance of complex pigments. The unique microstructure of the complex pigment provided pigments with good properties and high NIR reflectance. The optical properties must be obtained by using only 28.5% cobalt aluminate blue, which would substantially reduce the use of cobalt and hence, lead to a decrease in the cost of pigments. This would potentially reduce the environmental problems.
Conclusions
The complex pigments were obtained by calcination of the precursors with the precipitates of Al3+ and Co2+ on the surface of rutile TiO2. The resulting complex pigments consisted of rutile TiO2 and spinel CoAl2O4. The complex pigments exhibited good colour properties due to the visible absorption of the outer CoAl2O4 layer. These showed enhanced NIR reflectance because of the high NIR reflection of the inner rutile TiO2 core. The complex pigments may help reduce the use of cobalt. Furthermore, the complex pigments are environmentally friendly and can be produced at a relatively lower cost.Acknowledgements
The work described in this paper was supported by a grant from “China Postdoctoral Science Foundation funded project (2013M531925)” and “Fundamental Research Funds for the Central Universities (XDJK2014C038)”.Notes and references
- W. D. Li, J. Z. Li and J. K. Guo, J. Eur. Ceram. Soc., 2003, 23, 2289 CrossRef CAS.
- M. Zayat and D. Levy, Chem. Mater., 2000, 12, 2763 CrossRef CAS.
- X. L. Duan, M. Pan, F. P. Yu and D. R. Yuan, J. Alloys Compd., 2011, 509, 1079 CrossRef CAS PubMed.
- F. L. Yu, J. F. Yang, J. Y. Ma, J. Du and Y. Q. Zhou, J. Alloys Compd., 2009, 468, 443 CrossRef CAS PubMed.
- S. Salem, Mater. Lett., 2005, 139, 498 CrossRef PubMed.
- M. Jafari and S. A. Hassanzadeh-Tabrizi, Powder Technol., 2014, 266, 236 CrossRef CAS PubMed.
- S. N. Masoud, F. K. Masoud and D. Fatemeh, J. Sol-Gel Sci. Technol., 2009, 52, 321 CrossRef.
- D. M. A. Melo, J. D. Cunha, J. D. G. Fernandes, M. I. Bernardi, M. A. F. Melo and A. E. Mar-tinelli, Mater. Res. Bull., 2003, 38, 1559 CrossRef CAS.
- W. Z. Lv, Q. Qiu, F. Wang, S. H. Wei, B. Liu and Z. K. Luo, Ultrason. Sonochem., 2010, 17, 793 CrossRef CAS PubMed.
- Z. Z. Chen, E. Shi, W. J. Li, Y. Q. Zheng and W. Z. Zhong, Mater. Lett., 2002, 55, 281 CrossRef CAS.
- I. Mindru, G. Marinescu, D. Gingasu, L. Patron, C. Ghica and M. Giurginca, Mater. Chem. Phys., 2010, 122, 491 CrossRef CAS PubMed.
- M. Peymannia, A. Soleimani-Gorgani, M. Ghahari and F. Najafi, J. Eur. Ceram. Soc., 2014, 34, 3119 CrossRef CAS PubMed.
- D. Rangappa, T. Naka, A. Kondo, M. Ishii, T. Kobayashi and T. Adschiri, J. Am. Chem. Soc., 2007, 129, 11061 CrossRef CAS PubMed.
- C. Feldmann, Adv. Mater., 2001, 13, 1301 CrossRef CAS.
- S. Akdemir and E. Suvaci, Ceram. Int., 2011, 37, 863 CrossRef CAS PubMed.
- R. L. P. Berdahl and H. Akbari, Sol. Energy Mater. Sol. Cells, 2005, 89, 351 CrossRef PubMed.
- A. Libbra, L. Tarozzi, A. Muscio and M. A. Corticelli, Opt. Laser Technol., 2011, 43, 394 CrossRef CAS PubMed.
- L. Kai, N. M. N. Uemoto and V. M. J. Sato, Energ. Build., 2010, 42, 17 CrossRef PubMed.
- Z. N. Song, J. Qin, J. Qu, J. R. Song, W. D. Zhang, Y. X. Shi, T. Zhang, X. Xue, R. P. Zhang, H. Q. Zhang, Z. Y. Zhang and X. Wu, Sol. Energy Mater. Sol. Cells, 2014, 125, 206 CrossRef CAS PubMed.
- G. M. Revel, M. Martarelli, M. Emiliani, A. Gozalbo, A. Katsiapi, M. Taxiarchou, I. Arabatzis, I. Fasaki and S. He, Sol. Energy, 2014, 105, 770 CrossRef PubMed.
- J. Zou, P. Zhang, C. Liu and Y. Peng, Dyes Pigm., 2014, 109, 113 CrossRef CAS PubMed.
- A. S. T. Karlessi, N. G. M. Santamouris, D. N. Assimakopoulos and C. Papakatsikas, Build. Environ., 2011, 46, 38 CrossRef PubMed.
- S. Sangeetha, R. Basha, K. J. Sreeram, S. N. Sangilimuthu and B. U. Nair, Dyes Pigm., 2012, 94, 548 CrossRef CAS PubMed.
- S. F. Sameera, P. P. Rao, L. S. Kumari, V. James and S. Divya, Chem. Lett., 2013, 42, 521 CrossRef CAS.
- L. S. Kumari, P. P. Rao, A. N. P. Radhakrishnan, V. James, S. Sameera and P. Koshy, Sol. Energy Mater. Sol. Cells, 2013, 113, 134 CrossRef PubMed.
- S. Sameera, P. P. Rao, V. James, S. Divya and A. K. V. Raj, Dyes Pigm., 2014, 104, 41 CrossRef CAS PubMed.
- L. Liu, A. Han, M. Ye and M. Zhao, Sol. Energy Mater. Sol. Cells, 2015, 132, 377 CrossRef CAS PubMed.
- V. S. Vishnu and M. L. Reddy, Sol. Energy Mater. Sol. Cells, 2011, 95, 2685 CrossRef CAS PubMed.
- M. Zhao, A. Han, M. Ye and T. Wu, Sol. Energy, 2013, 97, 350 CrossRef CAS PubMed.
- S. Jose, A. Prakash, S. Laha and M. L. Reddy, Dyes Pigm., 2014, 107, 118 CrossRef CAS PubMed.
- Z. Tao, W. Zhang and Y. Huang, Solid State Sci., 2014, 34, 78 CrossRef CAS PubMed.
- S. Jose and M. L. Reddy, Dyes Pigm., 2013, 98, 540 CrossRef CAS PubMed.
- R. L. P. Berdahl and H. Akbar, Sol. Energy Mater. Sol. Cells, 2005, 89, 351 CrossRef PubMed.
- L. K. C. Souza, J. R. Zamian, G. N. R. Filho, L. E. B. Soledade, I. M. G. Santos, A. G. Souza, T. Scheller, R. S. Angélica and C. E. F. Costa, Dyes Pigm., 2009, 81, 187 CrossRef PubMed.
- L. Jiang, X. Xue, J. Qu, J. Qin, J. Song, Y. Shi, W. Zhang, Z. Song, J. Li, H. Guo and T. Zhang, Sol. Energy Mater. Sol. Cells, 2014, 130, 410 CrossRef CAS PubMed.
- X. Xue, J. Qin, J. Song, J. Qu, Y. Shi, W. Zhang, Z. Song, L. Jiang, J. Li, H. Guo and T. Zhang, Sol. Energy Mater. Sol. Cells, 2014, 130, 587 CrossRef CAS PubMed.
- R. Levinson, H. Akbari, P. Berdahl, K. Wood, W. Skilton and J. Petersheim, Sol. Energy Mater. Sol. Cells, 2010, 94, 946 CrossRef CAS PubMed.
- H. R. Hedayati, A. A. Sabbagh Alvani, H. Sameie, R. Salimi, S. Moosakhani, F. Tabatabaee and A. Amiri Zarandi, Dyes Pigm., 2015, 113, 588 CrossRef CAS PubMed.
- J. Zou, Dyes Pigm., 2013, 97, 71 CrossRef CAS PubMed.
- S. Cava, S. M. Tebcherania, S. A. Pianaro, C. A. Paskocimas, E. Longo and J. A. Varela, Mater. Chem. Phys., 2006, 97, 102 CrossRef CAS PubMed.
- M. A. Gabal, S. A. Hameed and A. Y. Obaid, Mater. Charact., 2012, 71, 87 CrossRef CAS PubMed.
- M. K. Yadav, A. V. Kothari and V. K. Gupta, Dyes Pigm., 2011, 89, 149 CrossRef CAS PubMed.
- M. Sales, C. Valentín and J. Alarcón, J. Eur. Ceram. Soc., 1997, 17, 41 CrossRef CAS.
- W. Wang, Z. Xie, G. Liu and W. Yang, Cryst. Growth Des., 2009, 9, 4373 CAS.
- L. G. A. Water, G. L. Bezemer, J. A. Bergwerff, M. Versluijs-Helder, B. M. Weckhuysen and K. P. Jong, J. Catal., 2006, 242, 287 CrossRef PubMed.
- L. F. Liotta, G. Pantaleo, A. Macaluso, G. D. Carlo and G. Deganello, Appl. Catal., A, 2003, 245, 167 CrossRef CAS.
- A. Synnefa, M. Santamouris and K. Apostolakis, Sol. Energy, 2007, 81, 488 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |